Figure 3.
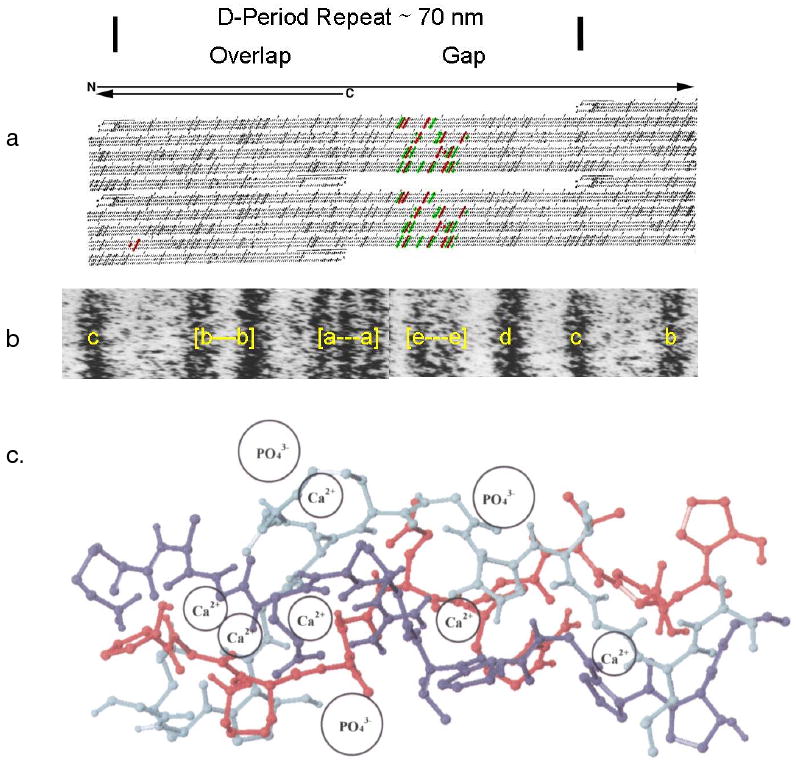
Correlation of collagen packing and sequence in the gap and overlap segments of 1 D period. a. Schematic showing charged residues as dark tilted stripes across all three chains, the segment correlated, in b, with the EM e-band, shown in the positively labeled 1 D period of a fibril. c. A section of type I collagen triple helix with a proline and hydroxyproline deficient region, residues 414 to 424. According to Silver et al27 this is a flexible region capable of binding both Ca and PO4 ions, and changing the conformation of the helix, based on the use of the SYBYL molecular modeling program and energy minimization after the addition of the mineral ions at the sites proposed. a. and b. Modified and reprinted with permission from Reference 280 [Veis, Reviews in Mineralogy & Geochemistry, V. 54]. Copyright 2003 Mineralogical Society of America. c. Modified and reprinted with permission from Reference 27 [Silver, et.al. Biomacromolecules]. Copyright 2001 American Chemical Society.
