Abstract
Background
The objective of this study was to determine whether neonatal nasogastric enteral feeding tubes are colonised by the opportunistic pathogen Cronobacter spp. (Enterobacter sakazakii) and other Enterobacteriaceae, and whether their presence was influenced by the feeding regime.
Methods
One hundred and twenty-nine tubes were collected from two neonatal intensive care units (NICU). A questionnaire on feeding regime was completed with each sample. Enterobacteriaceae present in the tubes were identified using conventional and molecular methods, and their antibiograms determined.
Results
The neonates were fed breast milk (16%), fortified breast milk (28%), ready to feed formula (20%), reconstituted powdered infant formula (PIF, 6%), or a mixture of these (21%). Eight percent of tubes were received from neonates who were 'nil by mouth'. Organisms were isolated from 76% of enteral feeding tubes as a biofilm (up to 107 cfu/tube from neonates fed fortified breast milk and reconstituted PIF) and in the residual lumen liquid (up to 107 Enterobacteriaceae cfu/ml, average volume 250 μl). The most common isolates were Enterobacter cancerogenus (41%), Serratia marcescens (36%), E. hormaechei (33%), Escherichia coli (29%), Klebsiella pneumoniae (25%), Raoultella terrigena (10%), and S. liquefaciens (12%). Other organisms isolated included C. sakazakii (2%),Yersinia enterocolitica (1%),Citrobacter freundii (1%), E. vulneris (1%), Pseudomonas fluorescens (1%), and P. luteola (1%). The enteral feeding tubes were in place between < 6 h (22%) to > 48 h (13%). All the S. marcescens isolates from the enteral feeding tubes were resistant to amoxicillin and co-amoxiclav. Of additional importance was that a quarter of E. hormaechei isolates were resistant to the 3rd generation cephalosporins ceftazidime and cefotaxime. During the period of the study, K. pneumoniae and S. marcescens caused infections in the two NICUs.
Conclusion
This study shows that neonatal enteral feeding tubes, irrespective of feeding regime, act as loci for the bacterial attachment and multiplication of numerous opportunistic pathogens within the Enterobacteriaceae family. Subsequently, these organisms will enter the stomach as a bolus with each feed. Therefore, enteral feeding tubes are an important risk factor to consider with respect to neonatal infections.
Background
Recently, considerable attention has been directed at the microbiological safety of PIF [1,2]. This has primarily been due neonatal infections by C. sakazakii and Salmonella, which were associated with contaminated PIF [3-6]. These products are not sterile, but are expected to comply with international microbiological standards [7]. Other Enterobacteriaceae which have been isolated from PIF include Enterobacter cloacae, Klebsiella pneumoniae, K. oxytoca, E. hormaechei,Citrobacter freundii, and E. coli [8,9]. The FAO/WHO [1,2] categorised these organisms as 'causality plausible, but not yet demonstrated' with respect to their potential to cause neonatal illness through the ingestion of reconstituted PIF. Although these organisms are opportunistic pathogens, there have been no confirmed outbreaks in NICUs attributed to their presence in contaminated PIF. This in part may be related to misidentification and delays in investigation. For example, reinvestigation of a C. sakazakii outbreak on a NICU revealed the organisms were E. hormaechei [9]. In another NICU outbreak, the powdered infant formula was not analysed until after the last neonatal case and the original batch of infant formula would no longer have been available [3]. The FAO/WHO [1] proposed that the risk of bacterial infection from powdered infant formula could be reduced by reconstitution with water > 70°C, minimising the time between reconstitution and feeding (< 2 h), and by not storing reconstituted feed at ambient temperature. These recommendations are reiterated by WHO [10], and various regulatory bodies [11-13]. However, there was no consideration that the nasogastric enteral feeding tube may act as a site for bacterial colonisation as a biofilm. The tube will be between ambient (outer portion) and body temperature (inner portion), with regular additions of nutrients from the infant feed and in-place over sufficient time periods for bacterial multiplication. Previously, nasogastric feeding tubes in a nursing home have been shown to be a reservoir for E. coli and Klebsiella with extended spectrum β-lactamases (ESBL) [14]. Berthelot et al. [15] proposed a role of enteral feeding in the colonisation and infection of premature infants by K. oxytoca. In recent years there has been a rise in incidence of neonatal infections due to Enterobacteriaceae, and they are the predominant causative agents in NICU outbreaks [16-18]. Klebsiella spp. infections outnumber staphylococci infections, and Serratia spp. are the third most common causative pathogen [16]. Pathogenic strains of E. coli are one of the leading causes of neonatal meningitis and sepsis [19]. Neonates may be particularly prone to Gram negative infections as their innate immune cells have low responses to lipopolysaccharide (part of the Gram negative cell wall structure) and macrophage response [20].
Prior to weaning, the infant intestinal flora is influenced by the feeding regime [21]. The initial intestinal flora of infants who are breast fed are dominated by lactic acid bacteria and bifidobacteria, whereas the intestinal flora of formula fed infants is more diverse and dominated by the Enterobacteriaceae and Bacteroides spp. [22]. However, this is a generalisation, as in practice neonates may receive a mixed nutrient source regime for short periods according to their nutritional needs. This may include the use of thickeners to reduce reflux, and these details may not be sufficiently recorded for later analysis. Few studies have considered the neonatal nasogastric enteral feeding tube in NICUs acting as a site of bacterial colonisation, and any influence of the feeding regime. Mehall et al. [23] detected Staphylococcus epidermidis, S. aureus, Enterococcus faecalis, E. cloacae and K. pneumoniae at > 103 cfu/ml in 71/125 enteral tubes from infants > 4 months, and that necrotizing enterocolitis developed in 7 formula fed infants with tubes containing > 105 Gram negative bacteria/ml. This group also reported the isolation of methicillin-resistant S. aureus from infant enteral feeding tubes [24]. Therefore collating information on hospital feeding regimes, and microbial analysis of feeding tubes will considerably improve our knowledge and understanding of potential risk factors to neonates linked to enteral feeding. This study is important to identify locations of bacterial multiplication which might be of risk to neonates, especially in NICUs.
Results
Neonate feeding regime
A total of 129 nasogastric enteral feeding tubes were collected from two NICUs; 25 and 104 respectively. The neonates' age range was from < 1 wk to greater than 4 wk, with the major group (42%) being > 4 wk (See additional file 1). Four specific feeding regimes were identified; 'breast milk', 'fortified breast milk', 'ready to feed formula', and 'reconstituted PIF'. Additionally, a number of neonates were receiving more than one type of feed. These are described as receiving a 'mixed feeding regime'. This latter category is a heterogeneous population. For example, some neonates received breast milk and fortified breast milk, whereas others received breast milk and reconstituted PIF. A thickener was added to feeds to reduce reflux for neonates receiving fortified breast milk, ready to feed formula, reconstituted PIF, and mixed feed. Ten tubes were received from neonates that were 'nil by mouth' (see additional file 1). The frequency of feeding was primarily every 2 h for breast milk, whereas there were equal numbers of every 2 h and 3 h for those receiving ready to feed formula (see additional file 1). Eight neonates were fed ready to feed formula, reconstituted PIF, and mixed feed continuously. The enteral feeding tubes had been in place for various time periods; ranging from < 6 h (22%) to > 48 h (13%) (see additional file 1). The gastric pH was measured prior to feeding, and was between 1.5 and 6. The average pH ranged from 2.5 to 4.3 for breast milk and reconstituted PIF fed neonates, respectively (see additional file 1).
Microbiological analysis of enteral feeding tubes
Bacterial counts on tubes
Enterobacteriaceae were isolated from the majority (76%) of samples, and from all feeding regimes (see additional file 2). The lowest frequency of isolation was 52% of the tubes from breast milk fed neonates, whereas the others ranged from 78 to 88% for mixed feeding regime and reconstituted PIF (see additional file 2).
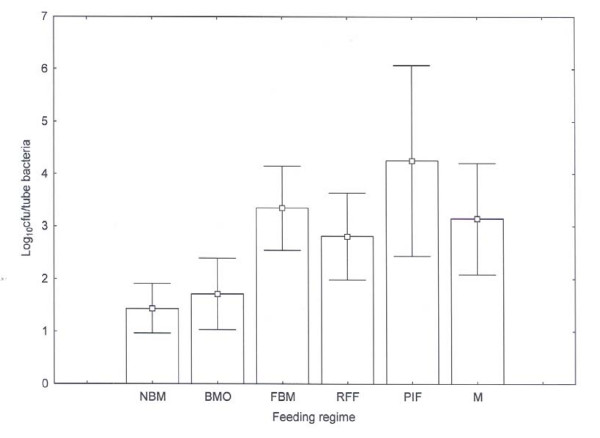
The dataset for NICU 2 (n = 104) was chosen for detailed statistical analysis due to the larger number of samples, and the 'nil by mouth' cohort was regarded as a control group for the feeding regimes. Feeding regime had a significant effect on the Enterobacteriaceae counts (F = 3.90, P < 0.001). The lowest values obtained were 'nil by mouth' and 'breast milk' only with an average values ca. 1.4 log10 cfu/tube (Fig. 1). The maximum 'nil by mouth' was 2.7 log10 cfu/tube which was less than the average for the remaining groups. The maximum for the breast milk cohort was 5.3 log10 cfu/tube. This value was considerable higher than the other neonates in the cohort, and could have been influenced by the exceptionally high pH (6.0) of this one sample. Fischer's protected least significant difference post-hoc tests suggested that 'fortified breast milk', 'ready to feed formula', 'reconstituted PIF' and 'mixed formula' all gave significantly greater counts than 'nil by mouth' regime. Hence, 'breast milk' and 'ready to feed' gave bacterial counts similar to those on the 'nil by mouth' regime. Statistical analysis showed that although there was a significant number of younger babies (< 1 wk) in this group, there was no statistical significant difference in colonisation between age groups (Analysis of variance 1-way F = 0.99, P > 0.05). Similarly, within the fortified breast milk group there was no significant effect of age on colonisation (Analysis of variance 1-way F = 0.89, P > 0.050). Hence, although there were some differences in age profiles within treatment groups and an overall effect of age, there was no evidence from our data that age effects colonization within a feeding group. It is accepted that the numbers within each group are small for these comparisons and it is possible that there were confounding problems of age with feeding regime. Within the mixed feeding regime group, analysis of the various feeding regimes suggested that those fed with breast milk and fortified breast milk, and PIF and breast milk, gave significantly higher counts than nil by mouth (F = 3.19, P < 0.05), but not the ready to feed formula and breast milk group. There was no difference in bacterial counts when a thickener was added to the feed.
Figure 1.
Enterobacteriaceae counts from biofilm material isolated from nasogastric enteral feeding tubes of neonates on various feeding regimes. Error bars indicate 95% confidence intervals. NBM = Nil by mouth (n = 10). BMO = Breast milk only (n = 17), FBM = fortified breast milk (n = 27), RFF = ready to feed formula (n = 21), PIF = Powdered infant formula (n = 8), M = mixed feeding regime (n = 20).
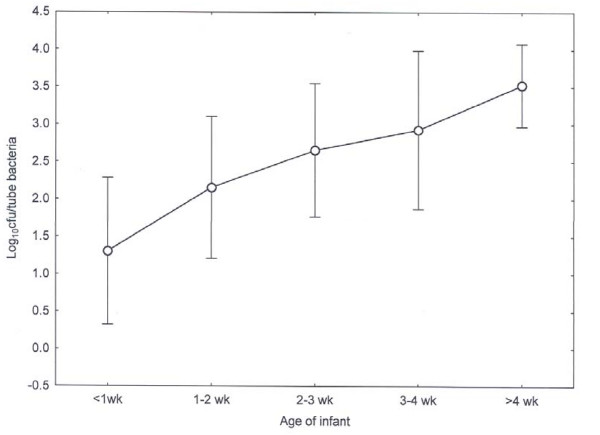
The effect of age of the infant on mean bacterial counts is shown in Fig. 2. There was a significant effect of age on bacterial counts when data were pooled (F = 4.49, P < 0.001) indicating a progressive increase in numbers with increasing age from 2 wk onwards. There was a significant effect of length of time the tube was in place when data were pooled (F = 6.91, P < 0.001). Compared with data at < 6 h, those at 6-12 h, 18- < 24 h, 24-48 h, and > 48 h, all had significantly greater bacterial counts, with maximum counts recorded at 48 h. When data are pooled, there was a positive but weak correlation between Enterobacteriaceae numbers and pH (r = 0.24, P < 0.05). However r2 suggests only 5.8% of the variance in bacterial numbers can be accounted for by pH. A one-way Anova comparing pH with age showed there was no significant difference in average pH between infant age classes. There were no significant effects of age within the 'nil by mouth' group (Analysis of variance 1-way F = 10.00 P > 0.05).
Figure 2.
Enterobacteriaceae counts from biofilm material on nasogastric enteral feeding tube according to age of neonate. Error bars indicate 95% confidence intervals.
Bacterial counts in residual liquids
During laboratory analysis of the feeding tubes, it was noted that there was residual liquid present. The average volume of residual liquid was 250 μl, and ranged between 30 and 400 μl. The average viable count was 107 cfu/ml, and ranged between < 102 to 108 cfu/ml. Therefore, the number of Enterobacteriaceae present in the residual liquid per tube was up to 107 cfu. This is the potential number of Enterobacteriaceae that would have entered the neonatal stomach with the next feed, if the tube had not been removed.
Bacterial species on tubes and in the residual liquids
The same Enterobacteriaceae species were isolated from both the residual liquids and the biofilms. The Enterobacteriaceae isolated were primarily E. cancerogenus (41%), S. marcescens (36%), E. hormaechei (33%), E. coli (29%), K. pneumoniae (25%), and R. terrigena (22%) (see additional file 2). Other organisms isolated less frequently included C. sakazakii from breast milk and ready to feed formula groups, and a single isolate of Y. enterocolitica from the reconstituted PIF group. E. cancerogenus, S. marcescens, and E. hormaechei and were isolated from all feeding regimes, including the 'nil by mouth' cohort (see additional file 2). The E. hormaechei and E. cancerogenus (identified by16S rDNA sequence analysis) were presumptively identified as E. cloaceae and K. oxytoca, respectively, by phenotypic profiling. Non-Enterobacteriaceae which were isolated from VRBGA included P. fluorescens, P. luteola and Chromobacterium violaceum. Electron microscopy of enteral feeding tube inner wall revealed that a dense, and morphologically diverse flora was present (Fig 3 and 4). This included a variety of short and long rod-shaped bacteria; some with tapering ends (Fig. 3). Yeast size cells were also visible (Fig. 4). Preliminary experiments with direct plating of enteral tube material on non-selective agar isolated staphylococci, lactic acid bacteria and Candida albicans (data not shown). Since these were not the focus of the study, they were not investigated further. There was no significant difference in the proportion of samples positive for Enterobacteriaceae between the feeding regimes (chi-square = 7.82, 5DF, P > 0.05). The distribution of bacterial species was different in tubes from 'nil by mouth' samples compared with all other regimes added together (chi-square = 16.28, 7DF P < 0.05). After removing the 'nil by mouth' samples from the statistical analysis, there were highly significant differences in the distribution of isolates between feeding regimes (chi-square = 94.95, 28DF, P < 0.001). Comparing each feeding regime with each of the others, showed that the breast milk and mixed feeding regimes were the only two giving similar distribution of isolates (chi-square = 9.72, 7DF, P > 0.05). Each of the other feeding regimes has a unique distribution of bacterial isolates.
Figure 3.

Electron microscopy of enteral feeding tube inner wall from neonate fed breast milk and ready to feed formula. Bar indicates 4 μm size marker.
Figure 4.

Electron microscopy of enteral feeding tube inner wall from neonate fed breast milk and reconstituted PIF with added thickener. Bar indicates 10 μm size marker.
Antibiotic resistance of isolated Enterobacteriaceae
All Enterobacteriaceae isolates were susceptible to gentamicin, ciprofloxacin and meropenem. The majority of strains were resistant to amoxicillin. The antibiograms for the remaining antibiotics are summarised in additional file 3. All S. marcescens isolates were resistant to amoxicillin and amoxicillin-clavulanic acid. Of note is the high frequency of resistance to ceftazidime (21% strains) and cefotaxime (23% strains) in E. hormaechei. Three of these strains contained ESBL. Four of the 37 E. coli strains were also resistant to ceftazidime and cefotaxime.
Discussion and Conclusion
In this study, a total of 129 nasogastric enteral feeding tubes with details of the neonates' feeding regime were obtained from 2 NICUs. The neonates were receiving a variety of feeds including expressed breast milk, reconstituted PIF, and sterile ready to feed formula. In addition tubes were received from infants that were 'nil by mouth'. The ages of the neonates varied with the feeding regime. Those on breast milk were predominantly < 1 wk, whereas those on fortified breast milk were > 4 wk (see additional file 1). Neonates receiving ready to feed formula were 1 to > 4 wk in age, whereas the majority of those on reconstituted powdered infant formula were > 4 wk in age. The frequency of feeding was primarily (54%) every 2 h, especially for those on breast milk, and secondly (24%) every 3 h. Eight neonates (6%) were fed continuously. This later practise is prone to temperature abuse, and has been linked with outbreaks of C. sakazakii in USA and France [3,25].
Enterobacteriaceae were isolated from the lumen and the inner wall of most (75%) enteral feeding tubes; see additional file 2. The organisms were initially identified using biochemical profiles and thereafter 16S rDNA sequence analysis as the later is more accurate [9,26]. The Enterobacteriaceae isolated were primarily E. coli, E. cancerogenus, E. hormaechei, K. pneumoniae, R. terrigena, and S. marcescens. These are well recognised opportunistic pathogens causing various gastrointestinal and respiratory diseases. Other organisms isolated included C. sakazakii, Y. enterocolitica, E. vulneris, and Pseudomonas spp. There were some differences in the flora between the feeding regimes, the reasons for which are currently unclear. The flora isolated from our neonatal samples is similar to that of Mehall et al. [24] who reported the isolation of E. cloacae, K. pneumoniae, S. maltophila and P. aeruginosa from enteral tubes of infants aged > 4 months. Other organisms present included Gram positive organisms such as staphylococci, and lactic acid bacteria, as well as Candida albicans. This fungus was also isolated in the study by Mehall et al. [24].
The antibiograms for the Enterobacteriaceae isolated are shown in additional file 3. Trimethoprim, ampicillin and co-amoxiclav are commonly used for minor infections in adults. Piptazobactam, amikacin, ceftazidime and cefotaxime are antibiotics that could be prescribed for empirical treatment of serious sepsis in infants on a neonatal intensive care unit. Of note is the high frequency of resistance by the E. hormaechei to the 3rd generation cephalosporins ceftazidime and cefotaxime. ESBL were detected in 3 of these strains. The antibiotic resistance patterns of the remaining strains could be due to derepressed chromosomal AMPC β-lactamase production [27]. As already proposed [28], it is plausible that the empiric use of antimicrobial agents selects for clones of EBSL organisms such as S. marcescens and K. pneumoniae. Although no link was established with feeding tube isolates, it is notable that these two species were also responsible for neonatal infections in both NICUs during our study. Resistance to these antibiotics would not be recognised until 24 - 48 h of culturing for the causative agent, during which time an ineffective antibiotic may have been used to treat the ill neonate. This delay in effective treatment could have serious consequences.
The 'nil by mouth' samples received during the study were treated as negative controls for the feeding regime comparison. They demonstrated that sterilisation of the outer tube surface effectively removed any oral-pharynx flora contamination. Therefore the organisms detected were deemed to originate from the inside of the enteral feed tube. It is probable that the few organisms isolated from these tubes originated from the throat by tracking along the outside of the tube into the stomach, or were residual organisms from before feeding stopped. Due to respect for strict patient confidentiality, all neonates were anonymous and hence we have no knowledge regarding the feeding regime of neonates prior to the sampling period. This unfortunately restricts our interpretation of data obtained for neonates that were 'nil by mouth' at the time of sample collection. Nevertheless, only low numbers of Enterobacteriaceae (< 3 log10 cfu/tube) were recovered from these samples (Fig. 1).
The Enterobacteriaceae were isolated from biofilms inside enteral feeding tubes of neonates who received only breast milk, but the numbers were lower than other feeding regimes (Fig. 1). Breast milk is not sterile, but does contain antibacterial agents such as maternal antibodies, lactoferrin, and lysozyme. Additionally the standard practice at the 2 NICUs was for expressed breast milk to be kept at 2-4°C for no more than 48 h and therefore very little bacterial growth could have occurred prior to feeding. Feeding tubes from neonates being fed fortified breast milk contained higher numbers of Enterobacteriaceae than unfortified breast milk; 3.6 log10 cfu/tube compared with 1.4 log10 cfu/tube respectively (Fig. 1). Human milk fortifiers may enable bacterial growth by providing free iron which is otherwise unavailable due to chelation in unfortified breast milk [29]. Another factor which may have affected the bacterial counts is that neonates fed fortified breast milk were older than those fed breast milk (see additional file 1). Some of the enteral tube flora could have been due to reflux of small intestinal contents into the stomach. Since older neonates will have a more established intestinal flora, increased bacterial numbers would be recovered from enteral tubes in the stomach. The 37 fortified breast milk tube samples were treated as one cohort since to our knowledge only one source of human milk fortifier was in use.
An unexpected result was the recovery of Enterobacteriaceae biofilms in enteral feeding tubes from 81% of neonates receiving sterile ready to feed formula. These products are sterilised inside glass jars by the manufacturer and have tamper-proof lids which would indicate any bacterial growth before use. These feeds were used directly from the sterile jar, and were not kept open for any length of time at temperatures enabling bacteria from extrinsic contamination to multiply. An alternative source of the enteral tube flora was the throat due to gastroesophageal reflux. This is common in preterm neonates, occurring 3-5 times per hour [30,31]. It occurs when the lower oesophageal sphincter relaxes, and this may increase the exposure of the feeding tube to the throat flora.
The highest Enterobacteriaceae biofilm levels were from enteral feeding tubes of neonates receiving reconstituted PIF; average 4.2 log10 cfu/tube. We have no knowledge regarding the range of PIF products being used on the wards, but it is reasonable to assume that various products had been prescribed by the neonatologists. However requesting further nutritional information was not permissible with respect to patient confidentiality. Therefore all neonates receiving reconstituted PIF were considered as one cohort. The same Enterobacteriaceae species were isolated as per other feeding regimes; E. coli, E. cancerogenus, R. terrigena, and S. liquifaciens; see additional file 2. Other Enterobacteriaceae isolated were Y. enterocolitica, K. ozaena and C. violaceum. Whether these Enterobacteriaceae originated from the powdered formula or reflux from the gastrointestinal tract is uncertain as no bacteriological analysis of the powdered formula was undertaken. Nevertheless the PIF were reconstituted at room temperature and therefore were not subject to hot water (> 70°C) to reduce the number of any intrinsic bacteria as recommended by the FAO/WHO [1,2]. Since, unlike human breast milk, there are no antibacterial agents in PIF any contaminating bacteria would be able to multiply in the formula while the tube was in place for up to 48 h.
As the bacterial biofilms age, the Enterobacteriaceae will break off in clumps. These clumps will inoculate any fresh feed in the tube lumen leading to further bacterial multiplication, and will subsequently enter the neonate stomach. Although the adult stomach is normally highly acidic, and kills the majority of ingested bacteria, this is not true for the neonate. The gastric pH was 2.5 (breast milk) and 3.5 to 4.3 for the remaining feeding regimes; see additional file 1. Edelson-Mammel et al. [32] have shown the acid-sensitivity of C. sakazakii. In their study of 12 strains, the viability decreased by 1 - 3.5 log cycles at pH 3.5 over a 5 h period. Koutsoumanis and Sofos [33] reported that the viable counts of E. coli O157:H7, Listeria monocytogenes, and Salmonella Typhimurium decreased by 2, 4 and 7 log cycles respectively when subjected to the same conditions. It is plausible that gastric juices were sometimes present in the enteral feeding tube which could reduce the biofilm formation. This may account for the absence of Enterobacteriaceae in tubes from neonates in which the pH was as low as 1.5 (see additional file 1).
Although C. sakazakii is well known for its association with infections of low-birth weight, preterm babies through contaminated PIF, in this study it was isolated from the enteral feeding tubes of two neonates receiving breast milk and ready to feed formula, respectively. C. sakazakii was the sole isolate from the breast milk tube, and was co-isolated with E. cancerogenus from the ready to feed formula tube. It should be noted that previously a C. sakazakii neonatal infection has been associated with breast milk [34]. Clinical isolates of Cronobacter can also produce profuse capsular material which may contribute to biofilm formation [3]. Non-Enterobacteriaceae isolated included P. fluorescens and P. luteola; see additional file 2. These organisms are well known for their ability to form biofilms which could entrap other organisms less able to colonise the tubing wall. Therefore their presence may enhance multiorganism biofilm formation.
The average Enterobacteriaceae count in the tube lumen was 107 cfu/ml, and the average residual liquid volume was 250 μl. Therefore, the number of Enterobacteriaceae present in the lumen was in the range from 102 to 107 cfu. This equates to the number of Enterobacteriaceae that would have entered the neonatal stomach with the next feed. The organisms probably originate from the attached bacterial biofilm, and therefore a reduction in biofilm formation should lead to lower numbers of organisms ingested via the lumen contents. The presence of the residual liquid was independent of the feeding regime, and reflected general feeding practices. The presence of the liquid was unexpected as normal practice is to flush the tube after feeding with a small volume of air or water to clear it. This residual liquid is a potential risk factor for neonatal infection that could be reduced by changing feeding practices in the NICU. There was limited opportunity for bacterial multiplication in the feed at room temperature during the feeding period (< 30 min) compared with the tube at 37°C which can be in place for > 48 h (see additional file 1). Therefore the enteral feeding tube could be a very important source of bacteria entering neonates, and would act as a significant amplifying step for opportunistic intestinal pathogens. These organisms would have entered the stomach as a bolus with the enhanced acid tolerance enabling them to survive the gastric acid and subsequently greater potential to colonise and infect the neonate.
The microbiological safety of neonatal feeds should not be exclusively focussed on reconstituted PIF due to C. sakazakii, but also on the general preparation and practices of enteral feeding to reduce the risk of exposure to other Enterobacteriaceae some of which may carry antibiotic resistance factors. Therefore, the practice of prolonged placement of enteral feeding tubes in neonates needs to be considered with respect to the increased risk of exposure to bacterial pathogens.
Methods
Preparation and administration of neonatal feeds
Powdered infant formula was reconstituted with sterile cold water at room temperature in a sterile bottle. Ready to feed formula was kept in the original bottle. Expressed breast milk (EBM) was obtained using a sterile expressing kit into sterile plastic pots. Fresh EBM was kept for up to 48 hours in a dedicated fridge at 2-4°C. Any EBM which was not to be used as fresh was frozen for up to 3 months in a dedicated freezer at -20°C. When required EBM was defrosted in the fridge and kept for up to 12 hours after removal from the freezer. The neonates were bolus or continuously fed via a nasogastric feeding tube composed of phthalate free PVC (gauge 3.5). Feeds were administered by pouring into a sterile syringe (without plunger) that was attached to the tube, and allowed to flow into the stomach by gravity. Duration of feeding was less than 30 minutes. Occasionally feeds were given by continuous infusion, and the syringe would then be changed every 4 hours.
Neonatal intensive care unit infections
During the period of sample collection, there were 38 episodes of neonatal infections in NICU 1 and 13 in NICU 2. In NICU 1, 10 infections were due to Enterobacteriaceae; 1 E. cloacae, and 2 K. oxytoca, 3 K. pneumoniae, and 4 E. coli. Whereas in NICU 2, 5 infections were due to Enterobacteriaceae; 1 E. coli, 2 K. pneumoniae, and 2 S. marcescens. The remaining infections in both units were primarily attributed to coagulase negative staphylococci.
Microbiological analysis
Nasogastric enteral feeding tubes were collected, without pre-selection, over a period of 11 months by nurses as part of their routine care of neonates in intensive care. The tubes were placed in sterile bags, and refrigerated at 5°C until analysis (max. 24 h). The outside of the tubes were sterilized with isopropyl alcohol. Any residual liquid in the tube lumen was flushed into a pre-weighed sterile Eppendorf tube, and the volume determined by weight difference. Using aseptic techniques, the tubing was cut into 2 cm lengths and except for the gastric 2 cm end, placed in 5 ml sterile saline in a conical test tube. The tubes were vortex mixed for 1 min, and then ultrasonicated at 40 kHz for 5 min at room temperature. The tubes were further vortex mixed for 1 min, and decanted into a sterile test tube. The procedure was repeated, and the combined saline rinses were centrifuged in a benchtop centrifuge (2400 g, 10 min). Afterwards, the supernatant was discarded and the bacterial pellet was resuspended in 1 ml sterile saline. The cell suspension was decimally diluted, and 100 μl volumes were spread on Violet Red Bile Glucose agar (VRBGA) plates (LabM, UK). The plates were incubated at 37°C, for up to 48 hours. Enterobacteriaceae colonies (red 1-2 mm diameter, usually surrounded by a reddish zone) were counted and representative colony types were subcultured on Tryptone Soya Agar (TSA) plates (Merck, Germany). Isolates were initially identified using phenotypic profiles with ID32 E (bioMerieux), and confirmed using 16S rDNA gene sequence analysis (Accugenix, Delaware, USA).
Nucleotide sequence accession numbers
The GenBank accession numbers of the E. cancerogenus and E. hormaechei isolates sequenced in this study are FM883655 to FM883666.
Antibiotic sensitivity testing
The susceptibilities of Enterobacteriaceae isolates to antimicrobial agents were determined by breakpoint on antibiotic supplemented Iso-Sensitest agar (catalog no. CM0471; Oxoid Ltd.) as according to the British Society for Antimicrobial Chemotherapy protocol [35]. The antibiotics tested were amikacin, gentamicin, amoxicillin, cefotaxime, cefuroxime, ceftazidime, ciprofloxacin, amoxicillin-clavulanic acid, gentamicin, meropenem, piperacillin-tazobactam, and trimethoprim (ADATAB; Mast Diagnostics, Bootle, United Kingdom). ESBL production was detected using the combination disc method as described in Health Protection Agency QSOP 51 [36] using ceftazidime-clavulanic acid, cefotaxime clavulanic acid, and cefpodoxime-clavulanic acid combination discs in comparison to individual-antibiotic ceftazidime, cefotaxime, and cefpodoxime discs according to the manufacturer's instructions (Mast Diagnostics, Bootle, United Kingdom).
Feeding regime questionnaire
During tube collection, a questionnaire concerning the feeding regime was completed by the attendant nurse. The feeding regime, any addition of a thickening agent, age of neonate, duration the tube had been in place, frequency of feeding and stomach pH prior to last feed were recorded. The amount of information for each tube was limited in order to comply with patient confidentiality. No record of patient clinical condition was recorded. Consequently, the source of each tube was anonymous.
Electron microscopy
The neonatal enteral feeding tube cells were fixed using 3% gluteraldehyde prepared in a 0.1 M phosphate buffer. The tubes were cut into representative 1 cm lengths and dissected longitudinally to expose the inner surface. The tubes were then washed in phosphate buffer and post fixed in 1% (w/v) osmium tetroxide prepared in 0.1 M phosphate buffer, post fixed in 1% osmium tetroxide, dehydrated through a gradual series of alcohol's up to 100% alcohol and then treated with hexamethyldisilazane for 5 minutes. The air-dried tubes were then attached to aluminium stubs, sputter-coated with gold and examined using a Stereoscan S250 Mark III SEM at 10-20 KV.
Data analysis
The data were analysed using analysis of variance (ANOVA) (STATISTICA software, Statsoft Inc., 2300 East 14th St, Tulsa, Ok, 74104, USA). Subsequent comparisons between group means were made using Fisher's protected least significant difference (PLSD) post-hoc test.
Authors' contributions
EH, EK, ML and JC-B carried out the experiments, collated and analysed the data. AH and RA undertook the statistical analysis of the data. CS and JG supervised the collection of the enteral feeding tubes. SS liaised with hospital staff and University personnel. SF designed the study, was the principal investigator and supervised the project. SF drafted the manuscript. All authors revised the manuscript, and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Summary of neonates' age and feeding regimes. Collation of data of the neonates sampled, their feeding regimes and gastric pH values.
Isolation of Enterobacteriaceae from biofilms on nasogastric enteral feeding tubes of neonates receiving a range of feeding regimes. Identification of Enterobacteriaceae isolated from biofilms inside neonatal enteral feeding tubes collated according to the feeding regime of the neonate.
Antibiogram profile of Enterobacteriaceae isolated from neonatal nasogastric enteral feeding tubes. Antibiotic resistance and sensitivity profiles for the Enterobacteriaceae isolated from neonatal enteral feeding tubes.
Contributor Information
Edward Hurrell, Email: edward.hurrell@hotmail.com.
Eva Kucerova, Email: Eva.kucerova@ntu.ac.uk.
Michael Loughlin, Email: Michael.loughlin@ntu.ac.uk.
Juncal Caubilla-Barron, Email: Juncal.caubilla-barron@ntu.ac.uk.
Anthony Hilton, Email: a.hilton@aston.ac.uk.
Richard Armstrong, Email: r.a.armstrong@aston.ac.uk.
Craig Smith, Email: Craig.Smith@nuh.nhs.uk.
Judith Grant, Email: Judith.Grant@nuh.nhs.uk.
Shiu Shoo, Email: Shing.Soo@nuh.nhs.uk.
Stephen Forsythe, Email: stephen.forsythe@ntu.ac.uk.
Acknowledgements
The authors thank the staff at the two NICUs for their co-operation, and the financial support of Mead Johnson Nutrition Co. and Nottingham Trent University. The authors are grateful for the technical assistance of Renee Ali and Evrim Altuntas, Prof. Poets (University of Tuebingen) for constructive comments, Dr W. Cooley (Veterinary Laboratory Agency, Surrey) for the electron microscopy, and Graham White (QMC) for his technical assistance in antibiotic testing.
References
- Food and Agriculture Organization/World Health Organization (FAO/WHO) Meeting report, MRA series 6. World Health Organization, Geneva, Switzerland; 2004. Enterobacter sakazakii and other microorganisms in powdered infant formula.http://www.who.int/foodsafety/publications/micro/mra6/en/index.html Date last accessed: 8.02.09. [Google Scholar]
- Food and Agriculture Organization/World Health Organization (FAO/WHO) Second Risk Assessment Workshop. Meeting report, MRA series 10. World Health Organization, Geneva, Switzerland; 2006. Enterobacter sakazakii and Salmonellain powdered infant formula.http://www.who.int/foodsafety/publications/micro/mra10/en/index.html Date last accessed: 8.02.09. [Google Scholar]
- Caubilla-Barron J, Hurrell E, Townsend S, Cheetham P, Loc-Carrillo C, Fayet O, Prere M-F, Forsythe SJ. Genotypic and phenotypic analysis of Enterobacter sakazakii strains from an outbreak resulting in fatalities in a neonatal intensive care unit in France. J Clin Microbiol. 2007;45:3979–3985. doi: 10.1128/JCM.01075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe S. Enterobacter sakazakii and other bacteria in powdered infant milk formula. Maternal and Child Nutrition. 2005;1:51–58. doi: 10.1111/j.1740-8709.2004.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler P, Herrara S, Rodriquez J, Cascante J, Cabral R, Echeita-Sarriondia A, Mateo S. Nationwide outbreak of Salmonella enterica serotype Kedougou infection in infants linked to infant formula milk, Spain, 2008. Euro Surveill. 2008;13(35) doi: 10.2807/ese.13.35.18963-en. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18963 Date last accessed: 8.02.09. [DOI] [PubMed] [Google Scholar]
- Iversen C, Mullane M, McCardell B, Tall BD, Lehner A, Fanning S, Stephan R, Joosten H. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, andproposal of Cronobacter sakazakii gen. nov., comb. nov.,C. malonaticus sp. nov. C. turicensis, sp. nov.,C. muytjensii sp. nov., C. dublinensis sp. nov.,Cronobacter genomospecies 1, and of three subspecies.C. dublinensis sp. nov. subsp. dublinensis subsp.nov. C. dublinensis sp. nov. subsp. lausannensissubsp. nov., and C. dublinensis sp. nov. subsp. lactaridi subsp. nov. Intl J Syst Envirn Microbiol. 2008;58:1442–7. doi: 10.1099/ijs.0.65577-0. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission. Report of the thirty-ninth session of the Codex committee on food hygiene. New Delhi, India. 2008. http://www.codexalimentarius.net/download/report/686/al31_13e.pdf Date last accessed: 8.02.09.
- Muytjens HL, Roelofs-Willemse R, Jaspar GH. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J Clin Microbiol. 1988;26:743–746. doi: 10.1099/mic.0.2008/021980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend SM, Hurrell E, Caubilla-Barron J, Loc-Carrillo C, Forsythe SJ. Characterization of an extended-spectrum beta-lactamase Enterobacter hormaechei nosocomial outbreak, and other Enterobacter hormaechei misidentified as Cronobacter (Enterobacter) sakazakii. Microbiology. 2008;154:3659–3667. doi: 10.1099/mic.0.2008/021980-0. [DOI] [PubMed] [Google Scholar]
- WHO. Safe preparation, storage and handling of powdered infant formula guidelines. 2007. http://www.who.int/foodsafety/publications/micro/pif2007/en/index.html Date last accessed: 8.02.09.
- EFSA. Opinion of the Scientific Panel on Biological Hazards on a request from the Commission related to the microbiological risks in infant formulae and follow-on formulae. The EFSA Journal. 2004;113:1–34. [Google Scholar]
- EFSA. Opinion of the Scientific Panel on Biological Hazards on the request from the Commission for review of the opinion on microbiological risks in infant formulae and follow-on formulae with regard to Enterobacteriaceae as indicators. The EFSA Journal. 2007;444:1–2. doi: 10.1001/jama.281.6.517. [DOI] [Google Scholar]
- United Kingdom Food Standards Agency. Guidance onpreparing infant formula. Monday 13 February 2006. 2006. http://www.food.gov.uk/foodindustry/guidancenotes/labelregsguidance/infform07guide Date last accessed: 8.02.09.
- Wiener J, Quinn JP, Bradford PA, Goering RV, Nathan C, Bush K. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281:517–23. doi: 10.1086/501881. [DOI] [PubMed] [Google Scholar]
- Berthelot P, Grattard F, Patural H, Ros A, Jelassi-Saoudin H, Pozzetto B. Nosocomial colonisation of premature babies with Klebsiella oxytoca : probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect Control Hosp Epidemiol. 2001;22:148–51. doi: 10.1016/j.ajic.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Gastmeier MD, Loui A, Stamm-Balderjahn S. Outbreaks in neonatal intensive care units - They are not like others. Am J Infect Control. 2007;35:172–6. doi: 10.1136/bmj.329.7477.1277. [DOI] [PubMed] [Google Scholar]
- McGuire W, Clerihew L, Fowlie P. Infection in the preterm infant. Br Med J. 2004;329:1277–80. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17:638–680. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, Laptook A, Walsh M, Oh W, Hale E. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005;24:635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- Harmsen HJM, Wideboer-Veloo ACM, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastr Nutr. 2000;30:61–67. doi: 10.1053/jpsu.2002.34467. [DOI] [PubMed] [Google Scholar]
- Townsend SM, Forsythe SJ. In: 'Enterobacter sakazakii'. Farber JM, Forsythe SJ, editor. Chapter 3. ASM Press, Washington; 2008. The neonatal intestinal microbial flora, immunity, and infections. [Google Scholar]
- Mehall JR, Kite CA, Saltzman DA, Wallett T, Jackson RJ, Smith SD. Prospective study of the incidence and complications of bacterial contamination of enteral feeding in neonates. J Pediatric Surgery. 2002;37:1177–82. doi: 10.1053/jpsu.2002.33831. [DOI] [PubMed] [Google Scholar]
- Mehall JR, Kite CA, Gilliam CH, Jackson RJ, Smith SD. Enteral feeding tubes are a reservoir for nosocomial antibiotic-resistant organisms. J Pediatr Surg. 2002;37:1011–2. doi: 10.1001/jama.287.17.2204. [DOI] [PubMed] [Google Scholar]
- Himelright I, Harris E, Lorch V, Anderson M. Enterobacter sakazakii infections associated withthe use of powdered infant formula -Tennessee, 2001. JAMA. 2002;287:2204–2205. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PubMed] [Google Scholar]
- Clarridge JE. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17:840–863. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet R. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48:1–14. doi: 10.1016/j.jhin.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivaro V, Bagattini M, Salza MF, Raimondi F, Rossano F, Triassi M, Zarrilli R. Risk factors for extended-spectrum beta-lactamase-producing Serratia marcescens and Klebsiella pneumoniae acquisition in a neonatal intensive care unit. J Hosp Infect. 2007;67:135–41. doi: 10.1542/peds.100.2.240. [DOI] [PubMed] [Google Scholar]
- Jocson MA, Mason EO, Schanler RJ. The effects of nutrient fortification and varying storage conditions on host defense properties of human milk. Pediatrics. 1997;100:240–243. doi: 10.1542/peds.109.1.8. [DOI] [PubMed] [Google Scholar]
- Peter CS, Sprodowski N, Bohnhorst B, Silny J, Poets CF. Gastroesophageal reflux and apnea of prematurity. No temporal relationship. Pediatrics. 2002;109:8–11. doi: 10.1542/peds.113.2.e128. [DOI] [PubMed] [Google Scholar]
- Poets CF. Gastroesophageal reflux: a critical review of its role in preterm infants. Pediatrics. 2004;113:128–132. doi: 10.1111/j.1750-3841.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Edelson-Mammel SG, Porteous MK, Buchanan RL. Acidresistance of twelve strains of Enterobacter sakazakii and the impact of habituating the cells to an acidic environment. J Food Sci. 2006;71:M201–M207. doi: 10.1111/j.1472-765X.2004.01491.x. [DOI] [Google Scholar]
- Koutsoumanis KP, Sofos JN. Comparative acid stress response of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Typhimurium after habituation at different pH conditions. Lett Appl Microbiol. 2004;38:321–326. doi: 10.1111/j.1472-765X.2004.01491.x. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen N, Fanaroff AA, Lemons JA. Enterobacter sakazakii is a rare cause of neonatal septicemia or meningitis in VLBW infants. J Pediatr. 2004;144:821–3. doi: 10.1016/j.jpeds.2004.02.045. [DOI] [PubMed] [Google Scholar]
- British Society for Antimicrobial Chemotherapy. BSAC methods for antimicrobial susceptibility testing, version 7. 2008. http://www.bsac.org.uk/_db/_documents/version_7_1_february_2008.pdf Date last accessed: 8.02.09.
- United Kingdom Health Protection Agency. QSOP 51. Health Protection Agency, London, United Kingdom; 2006. Laboratory detection and reporting of bacteria with extended spectrum-lactamases. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of neonates' age and feeding regimes. Collation of data of the neonates sampled, their feeding regimes and gastric pH values.
Isolation of Enterobacteriaceae from biofilms on nasogastric enteral feeding tubes of neonates receiving a range of feeding regimes. Identification of Enterobacteriaceae isolated from biofilms inside neonatal enteral feeding tubes collated according to the feeding regime of the neonate.
Antibiogram profile of Enterobacteriaceae isolated from neonatal nasogastric enteral feeding tubes. Antibiotic resistance and sensitivity profiles for the Enterobacteriaceae isolated from neonatal enteral feeding tubes.