Table 1.
Phosphine-Mediated [3 + 3] Aziridine/Allene Annulationa
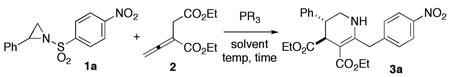 | ||||
|---|---|---|---|---|
| entry | PR3 | mol% | yield (%)b | dr (trans/cis)c |
| 1 | PBu3 | 20 | 0 | N/A |
| 2 | PPh3 | 20 | 15 | 10:1 |
| 3 | PPh3 | 50 | 37 | 9:1 |
| 4 | PPh3 | 100 | 73 | 9:1 |
| 5 | PPh3 | 200 | 61 | 9:1 |
| 6d | PPh3 | 100 | 63 | 9:1 |
| 7e | PPh3 | 100 | 48 | 9:1 |
| 8 | EtPPh2 | 100 | 2 | – |
| 9 | Et2PPh | 100 | 0 | N/A |
| 10 | P(NMe2)3 | 100 | 0 | N/A |
| 11 | P(OEt)3 | 100 | 0 | N/A |
All reactions were performed using 0. 1 mmol of 1a and 4.8 equiv of 2 in CH2Cl2 at rt for 72 h, unless otherwise specified.
Isolated yield after chromatographic purification.
Diastereoisomeric ratio determined through HPLC (internal standard: 2-bromopyridine).
1,2-Dichloroethane as solvent; other common organic solvents provided isolated product yields of less than 10%.
40 °C, 48 h.
