Table 2.
Syntheses of Tetrahydropyridinesa
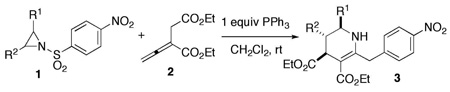 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | product | yield (%)b |
dr (trans/cis)c |
| 1 | H (1b) | 2-MeC6H4 | 3b | 88 | 90:10 |
| 2 | H (1c) | 3-MeC6H4 | 3c | 82 | 86:14 |
| 3 | H (1d) | 4-MeC6H4 | 3d | 64 | 89:11 |
| 4 | H (1e) | 2,4-Me2C6H3 | 3e | 82 | 81:19 |
| 5 | H (1f) | 2,5-Me2C6H3 | 3f | 98 | 92:8 |
| 6 | H (1g) | 4-FC6H4 | 3g | 76 | 88:12 |
| 7 | H (1h) | 2-ClC6H4 | 3h | 46 | 97:3 |
| 8 | H (1i) | 3-ClC6H4 | 3i | 86 | 83:17 |
| 9 | H (1j) | 4-ClC6H4 | 3j | 84 | 90:10 |
| 10 | H (1k) | 3-BrC6H4 | 3k | 58 | 85:15 |
| 11 | H (1l) | 4-BrC6H4 | 3l | 75 | 88:12 |
| 12 | H (1m) | 2-naphthyl | 3m | 58 | 85:15 |
| 13 | Me (1n) | H | 3n | 66 | 41:59 |
| 14 | H (1o) | H | 3o | 37 | N/A |
All reactions were performed using 0.1 mmol of the aziridine and 4.8 equiv of the allenoate.
Isolated yields.
Diastereoisomeric ratio determined using HPLC (internal standard: 2-bromopyridine).
