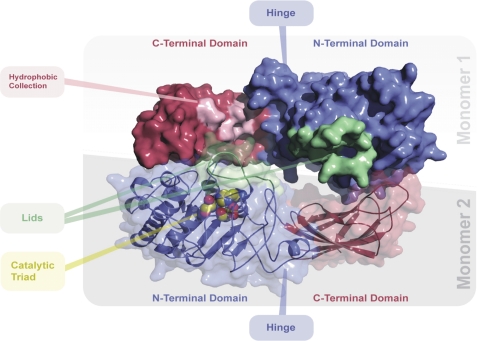
FIGURE 7.
Molecular model of the EL dimer. Top view of the human endothelial lipase dimer model is shown. The N-terminal “head” domains are shaded in blue; the C-terminal “tail” domains are shaded in red. The activation lid is colored green. The three catalytic residues, serine 169, aspartic acid 193, and histidine 274 are rendered in a space-filling format and located under the activation lid. A collection of exposed hydrophobic residues, Met-351, Leu-382, Pro-383, Ile-386, Val-387, Phe-398, Leu-399, Val-400, Pro-437, Pro-440, and Gly-441, is highlighted in pink on monomer 1. Monomer 2 is rendered so as to reveal the N- and C-terminal domains, hinge, and secondary structure.