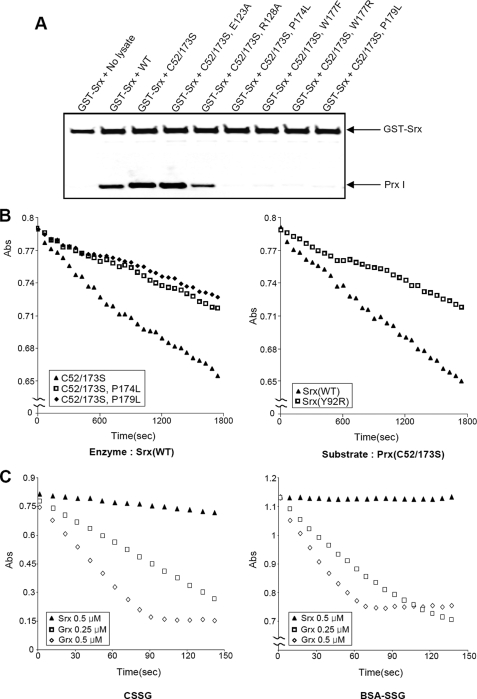
FIGURE 6.
A, the effect of Prx I mutations on interactions with Srx. Wild-type and mutant Prx I-expressing cell lysates (500 μg) were incubated for 2 h at 4 °C with GST-hSrx fusion protein (5 μg) in 1 ml of binding buffer (50 mm HEPES buffer (pH 7.0), 150 mm NaCl, l% Triton X-100, 1 mm EDTA, and 1 mm DTT). Samples precipitated with GSH-Sepharose resins were subjected to Western blot analysis with antibodies to Prx I or GST. B, deglutathionylation of Prx I mutants catalyzed by Srx or Srx (Y92R). The reaction rates were monitored by a coupled assay system as described in Fig. 3. In these reactions, 1 μm Srx or Srx(Y92R) was used to catalyze the deglutathionylation of 20 μm glutathionylated Prx I mutants. The data show the decrease in A340 nm due to NADPH oxidation by GSSG generated during the glutathionylation reaction. C, deglutathionylation assay with CSSG and BSA-SSG as substrates. The deglutathionylation of CSSG and BSA-SSG (100 μm each) was catalyzed by 0.5 μm Srx (▴), 0.25 μm human Grx I (□), or 0.5 μm human Grx I (◇). The reactions were monitored by the coupled assay system described in B. The reaction time courses were obtained at 37 °C.