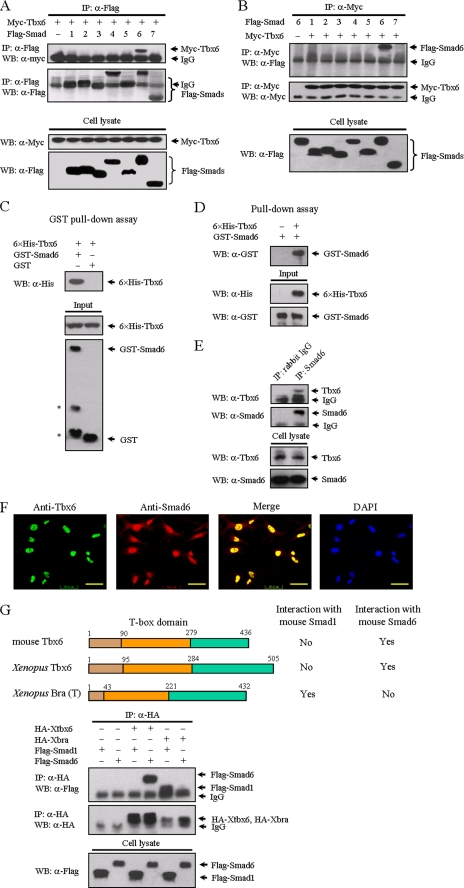
FIGURE 1.
Tbx6 interacts with Smad6. A, identification of Smad6 as a novel Tbx6-interacting protein. FLAG-tagged Smads 1–7 were individually co-transfected with Myc-tagged Tbx6 into HEK 293T cells as indicated. After transfection for 24 h, cells were lysed, immunoprecipitated (IP) with anti-FLAG antibody, and analyzed by Western blotting (WB) with anti-Myc antibody (top panels). Whole cell lysates were immunoblotted directly with anti-Myc or anti-FLAG antibodies to demonstrate the expression of Myc-Tbx6 and FLAG-Smads, respectively (bottom panels). B, reciprocal immunoprecipitation with anti-Myc was carried out. Only Smad6 was co-precipitated with Tbx6. C, Tbx6 directly binds to Smad6 in vitro. Bacterially expressed GST-Smad6 fusion protein was purified using glutathione-agarose beads and incubated with His6-Tbx6 fusion protein. Associated His6-Tbx6 protein was readily detected by immunoblotting (top panel). The asterisks denote degraded bands of GST-Smad6 fusion protein (bottom panel). An equimolar amount of GST was used as a negative control. D, purified His6-Tbx6 fusion protein was incubated with GST-Smad6 and precipitated with anti-His antibody. Associated GST-Smad6 protein was detected by Western blot. E, interaction between endogenous Tbx6 and Smad6 in TT-D6 cells. TT-D6 cell extracts were subjected to immunoprecipitation with anti-Smad6 polyclonal antibody. Normal rabbit IgG was used as a negative control. The immunocomplexes were analyzed by immunoblotting using anti-Tbx6 antibody. F, Tbx6 is co-localized with Smad6 in the nucleus. TT-D6 cells were fixed and stained with the indicated antibodies to determine the localization of Tbx6 (green) and Smad6 (red). Nuclei were stained with 4,6-diamidino-2-phenylindole. The bar represents 50 μm for each section. G, T-box proteins have distinct affinities for different Smad proteins. Top, schematic diagrams depict Tbx6 homologues in mouse and Xenopus. Their interactions with Smad1 or Smad6 from co-immunoprecipitation experiments are shown in the bottom panels. HA, hemagglutinin.