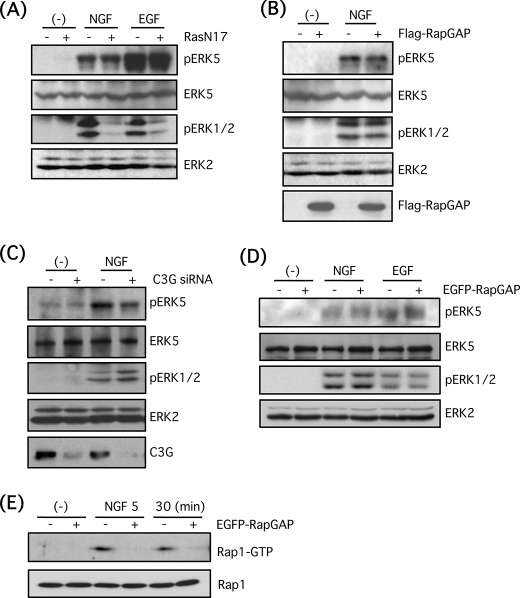
FIGURE 4.
Small G-proteins, Ras and Rap1, are not involved in ERK5 phosphorylation by NGF in PC12 cells. A, PC12 cells were infected with adenovirus encoding the dominant-negative mutant of Ras (RasN17) for 3 days at 50 m.o.i. Then the cells were stimulated with NGF (100 ng/ml) or EGF (100 ng/ml) for 5 min, and phospho-ERK5, total ERK5, phospho-ERK1/2, and total ERK2 were examined by Western blotting. B, PC12 cells were infected with adenovirus encoding FLAG-Rap1GAP1 for 3 days at 50 m.o.i. in the presence of transactivating virus (250 m.o.i.). Then the cells were stimulated with NGF (100 ng/ml) for 5 min, and phospho-ERK5, total ERK5, phospho-ERK1/2, total ERK2, and FLAG-Rap1GAP1 were examined by Western blotting. C, PC12 cells were transfected with double-stranded siRNA for C3G. After 3 days, the cells were stimulated with NGF (100 ng/ml) for 5 min, and phospho-ERK5, total ERK5, phospho-ERK1/2, total ERK2, and C3G were examined by Western blotting. D, EGFP-Rap1GAP-positive or -negative PC12 cells were stimulated with NGF (100 ng/ml) or EGF (100 ng/ml) for 5 min, and phospho-ERK5, total ERK5, phospho-ERK1/2, and total ERK2 were examined by Western blotting. E, EGFP-Rap1GAP-positive or -negative PC12 cells were stimulated with NGF (100 ng/ml) for 5 and 30 min, and Rap1 pulldown was performed as described under “Experimental Procedures.” Rap1-GTP and total Rap1 were examined by Western blotting.