FIGURE 6.
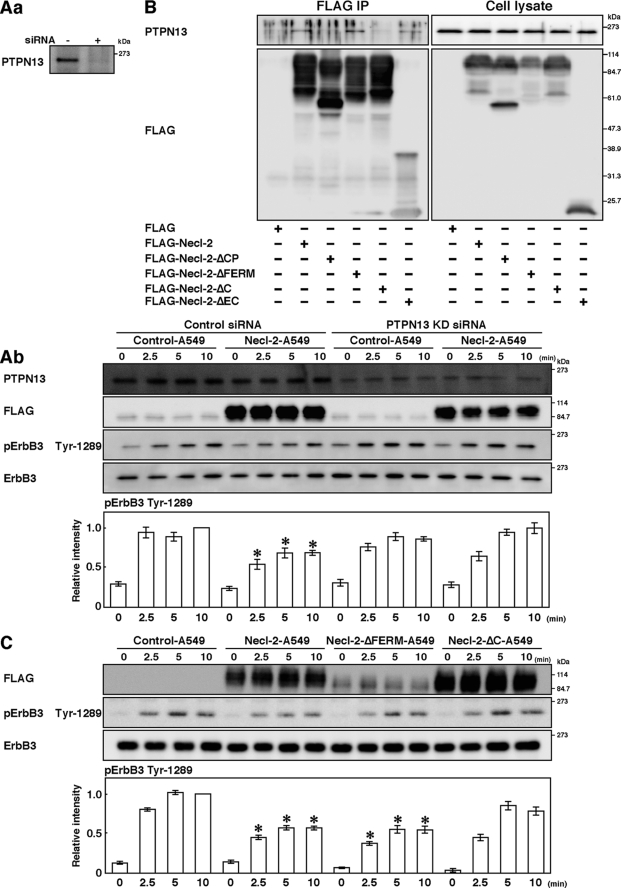
Involvement of PTPN13 in the inhibitory effect of Necl-2. A, knockdown of PTPN13. A549 cells were transfected with negative control stealth RNAi and stealth RNAi against PTPN13 (Control-A549 and PTPN13-KD-A549 cells, respectively). Aa, confirmation of knockdown of PTPN13. Samples were subjected to Western blotting using the anti-PTPN13 pAb. Ab, assay for the HRG-induced, ErbB2-catalyzed tyrosine phosphorylation of ErbB3. Cells were starved of serum for 20 h and stimulated by 10 ng/ml HRG for the indicated periods of time and lysed with RIPA buffer. Samples were subjected to Western blotting using the indicated Abs. Bars in the graph represent the relative band intensity of phosphorylated ErbB3, assessed as described in Fig. 3. Error bars indicate the means ± S.E. of three independent experiments. *, p < 0.02 versus Control-A549 cells. B, assay for the co-immunoprecipitation of PTPN13 with Necl-2. HEK293 cells were transfected with FLAG-Necl-2, FLAG-Necl-2-ΔCP, FLAG-Necl-2-ΔFERM, FLAG-Necl-2-ΔC, or FLAG-Necl-2-ΔEC. Each FLAG-Necl-2 mutant was immunoprecipitated using the anti-FLAG mAb, and samples were subjected to Western blotting using the indicated Abs. C, assay for the HRG-induced, ErbB2-catalyzed tyrosine phosphorylation of ErbB3 using Necl-2 mutants. A549 cells were transfected with empty vector, FLAG-Necl-2, FLAG-Necl-2-ΔFERM, or FLAG-Necl-2-ΔC expression vector (Control-A549, Necl-2-A549, Necl-2-ΔFERM-A549, or Necl-2-ΔC-A549 cells, respectively). Cells were starved of serum for 20 h and stimulated by 10 ng/ml HRG for the indicated periods of time. Samples were subjected to Western blotting using indicated Abs. Bars in the graph represent the relative band intensity of phosphorylated ErbB3, assessed as described in Fig. 3. Error bars indicate the means ± S.E. of three independent experiments. *, p < 0.01 versus Control-A549 cells. The results shown are representative of three independent experiments.