Figure 6. Small ensembles of Ndc80 complexes track with disassembling microtubule tips.
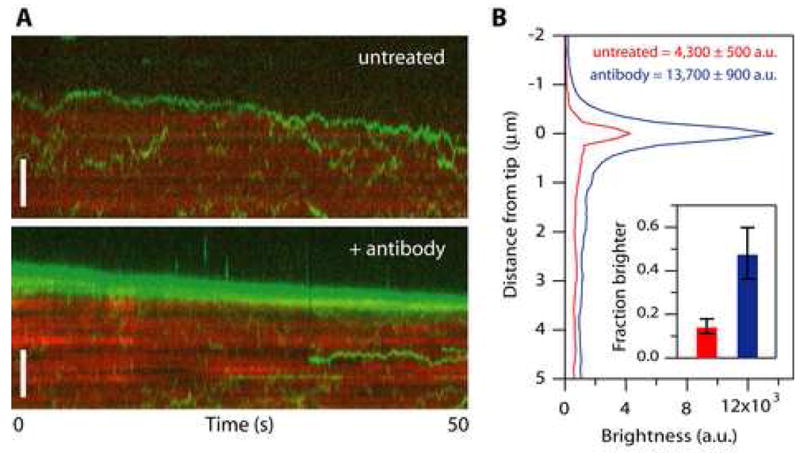
(A) Kymographs showing movement of particles composed of Ndc80 complexes (green) on disassembling microtubules (red). At 1 nM Ndc80 complex the microtubules were crowded with diffusing particles, many of which encountered the disassembling tip. No clear tip tracking was evident. However, in some cases the density of particles became noticeably higher at a disassembling tip relative to the lattice. When individual particles could be discerned as they encountered a disassembling tip, they sometimes appeared to ‘bounce’ repeatedly off the tip (upper kymograph). After pre-treating the Ndc80 complex with anti-His antibody to drive oligomerization, more disassembling tips accumulated fluorescent particles, and some long episodes of processive tip tracking were observed (lower kymograph). Scale bars, 3 μm.
(B) Profiles of average fluorescence versus position along the microtubule for a population of disassembling filaments decorated with Ndc80 complexes. Only intervals when the tip appeared to accumulate fluorescent particles were included in the averages. During these events, the disassembling tips became brighter if the Ndc80 complex had been pre-treated with antibody (blue curve, N = 21 events from 17 microtubules in 10 recordings) than without antibody pre-treatment (red curve, N = 24 events from 20 microtubules in 27 recordings). The fraction of disassembling tips that accumulated fluorescence was also increased after antibody pre-treatment (inset). Uncertainties in peak brightness represent s.d., and in the inset they represent counting error.
