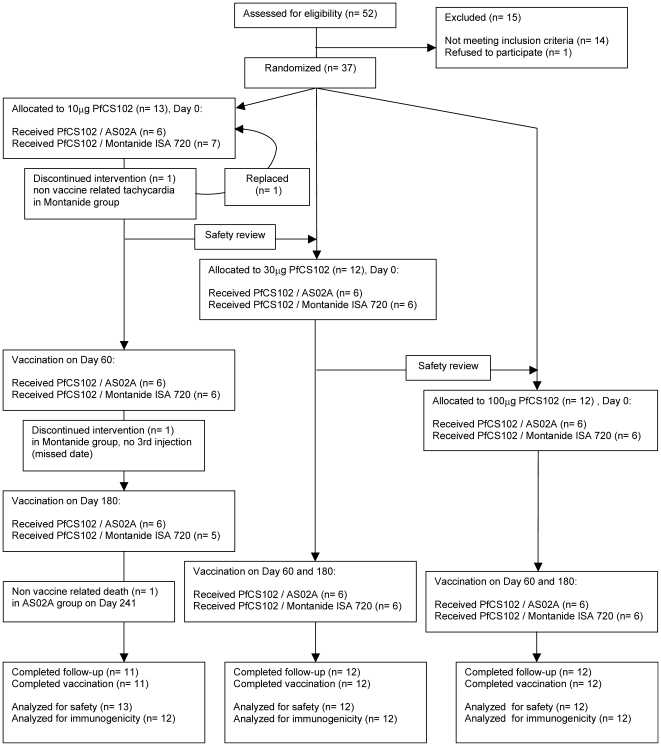
Figure 1. Study flow chart.
Design of the Phase I, double-blind, dose-ranging study of 3 vaccine dosing regimens combined to two different adjuvants AS02A and Montanide ISA 720. For each dose, volunteers were randomized by blocks of 12, with 6 individuals per adjuvant. Volunteers were seen 2 and 30 d after the first injection, 2 and 15 d after the second injection and 2, 15, 180, and 360 d after the third injection. A phone call was addressed to all volunteers at d 30 after the 2nd and 3rd injection.