Figure 2.
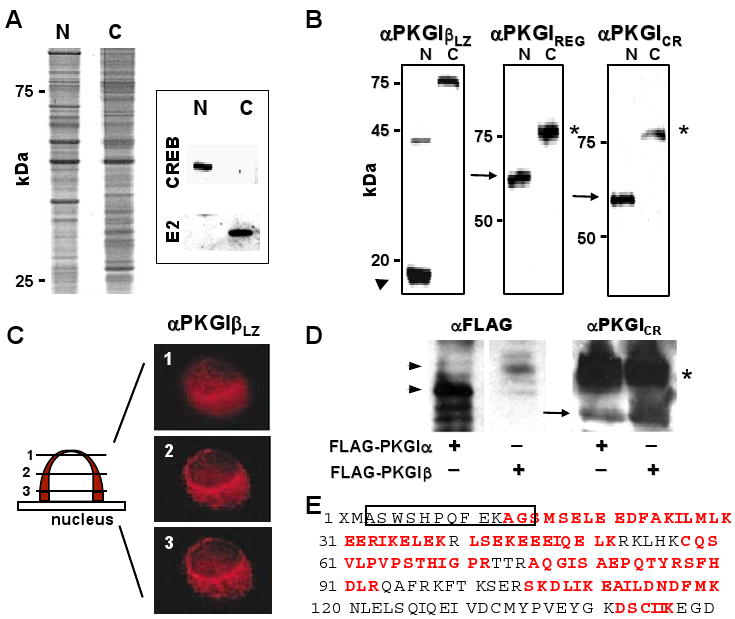
PKGI proteolytic cleavage in SMC. (A) Hypotonic cell shock and isopycnic density centrifugation yielded nuclear (N) and cytoplasmic (C) SMC protein fractions with differential protein abundance and CREB and pyruvate dehydrogenase E2 subunit (E2) immunoreactivity. (B) Purified A7r5 cell nuclear proteins contained a ∼18-kDa protein fragment (arrowhead) with PKGILZ immunoreactivity and a 60-kDa protein (arrow) with PKGIREG and PKGICR immunoreactivity. Although these PKGI fragments were not identified in the cytosolic fraction, full-length ∼78-kDa PKGI in the cytosol exhibited all of these immunoreactivities. (C) PKGIβ LZ domain immunoreactivity was identified in perinuclear regions in Z-dimension optical sections of A7r5 nuclei. (D) and (E) PKGIα was also fragmented in intact cells. NH2-terminal (arrowheads) and COOH-terminal fragments (arrow) of PKGIα and PKGIβ and full-length PKGI isoforms (*) were identified in lysates of BHK cells expressing FLAG-PKGIα and FLAG-PKGIβ. The apparent masses of the NH2-terminal fragments of the PKGI isoforms are consistent with the different sizes of the their LZ domains. Mass spectroscopy identified peptide portions (red letters) of the LZ domain of PKGIα and the precipitating SBP2 epitope (box).
