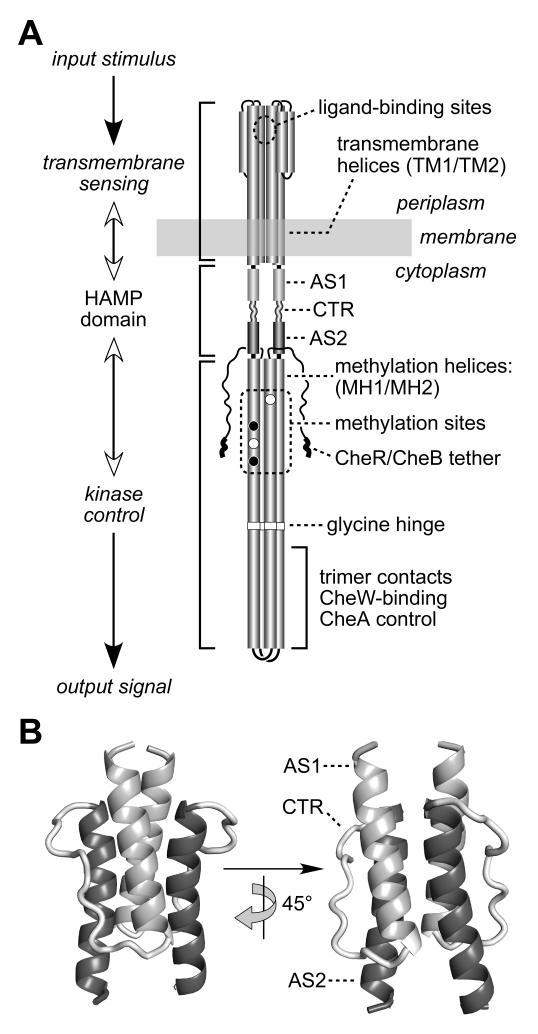
Fig. 1. Functional architecture of MCP molecules.
A) Schematic model of the Tsr homodimer. The CheR/CheB tether (NWETF pentapeptide) marks the C-terminus of each subunit. Helical segments are represented as thickened cylinders, whose relative lengths are approximately to scale. Each Tsr subunit has two transmembrane helices (TM1, TM2) and two methylation helices (MH1, MH2) containing four principal methylation sites; two (black circles) are synthesized as glutamines and subsequently deamidated to glutamates (white circles).
B) Modeled structure of the Tsr HAMP domain. Atomic coordinates were obtained by threading the Tsr HAMP sequence onto the Af1503 HAMP structure (Hulko et al., 2006). The AS1 helix is labeled at its N-terminus; the AS2 helix is labeled at its C-terminus; CTR is the connector segment joining the two helices.