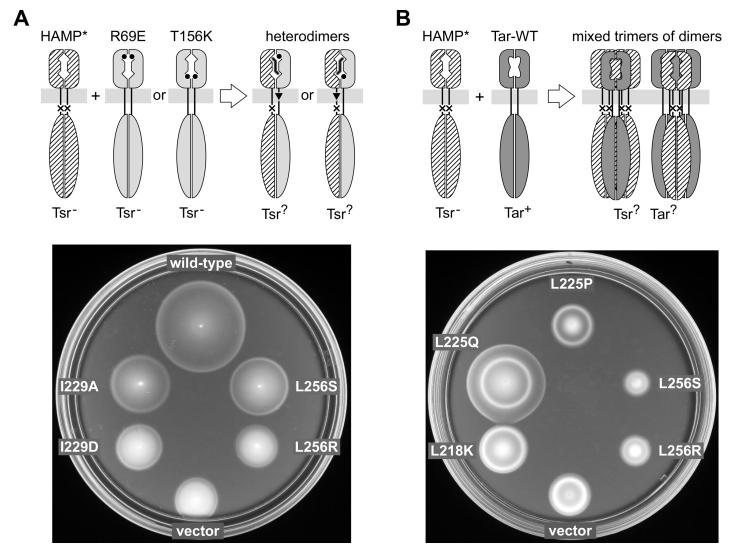
Fig. 4. Functional tests of Tsr-HAMP mutations.
A) Dominance and asymmetry test. Upper: Plasmid-encoded mutant Tsr-HAMP (HAMP*) subunits were expressed in a host strain with chromosomally-encoded Tsr-R69E or Tsr-T156K subunits, which have lesions at the serine-binding site. The resulting heterodimers have one functional binding site, which produces ligand-induced piston motions through the wild-type HAMP subunit (in the case of R69E) or through the mutant HAMP subunit (in the case of T156K). Lower: Examples of dominant and recessive Tsr-HAMP defects. UU2378 (tsr-T156K) cells containing pPA114 derivatives were tested for Tsr function on tryptone soft agar plates containing 12.5 μg/ml chloramphenicol and 0.6 μM sodium salicylate and incubated at 30°C for 7.5 hours. The wild-type control was pPA114; the vector control was pKG116. Tsr-HAMP mutants I229A and L256S are recessive; I229D and L256R are dominant.
B) Jamming and rescue test. Upper: Plasmid-encoded Tsr-HAMP* molecules were expressed in a tar+ Δtsr host strain. Tar and Tsr subunits do not form heterodimers, but do form mixed trimers of dimers, in which some Tsr defects regain function (rescue) and other Tsr defects spoil Tar function (jamming). Lower: Examples of rescuable and jamming Tsr-HAMP defects. UU1623 cells containing pPA114 derivatives were tested for Tsr function on tryptone soft agar plates containing 12.5 μg/ml chloramphenicol and 0.6 μM sodium salicylate and incubated at 32.5°C for 9 hours. The vector control was pKG116. L256S and L256R jam Tar; L225Q is rescuable (and does not jam Tar); L218K and L225P are neither rescuable nor jamming.