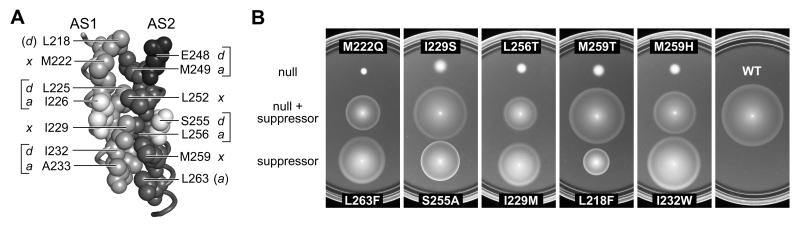
Fig. 5. Tsr-HAMP packing layers and example suppression pairs.
A) Side view of AS1-AS2 packing interactions in one subunit of the modeled 4-helix Tsr-HAMP bundle. Space-filled residues compose the packing interface. Critical AS1 residues are shown with light gray atoms); critical AS2 residues with either dark gray or black (E248) atoms. Noncritical residues at the packing interface (I226, S255) have white atoms. Brackets denote the four packing layers (x in one helix; a, d in the other). L218 (d) and L263 (a) participate in packing interactions at the top and bottom of the bundle, respectively.
B) Examples of suppression quality. Derivatives of pPA114 carrying a Tsr-HAMP null mutation alone, a suppressor mutation alone, and the double mutant combination were tested for Tsr function in receptorless host strain UU1250. Transformant cells were placed on tryptone soft agar plates containing 12.5 μg/ml chloramphenicol and 0.6 μM sodium salicylate and incubated at 30°C for 7 hours.