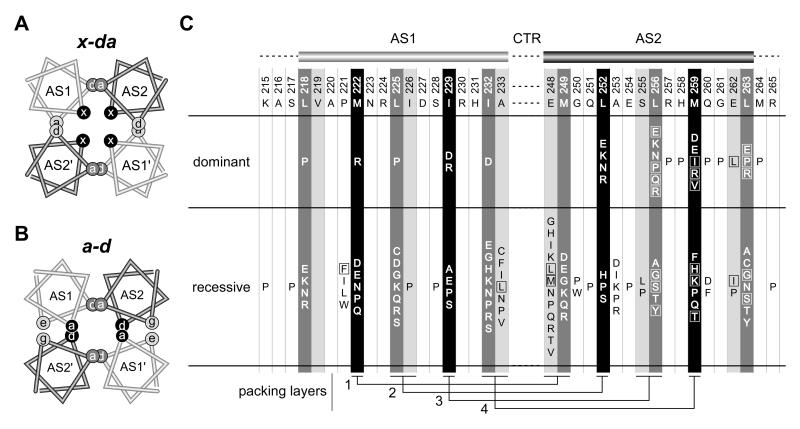
Fig. 7. Bundle arrangements and null lesions in the Tsr-HAMP AS1 and AS2 helices.
A) Helical wheel cross-section of the x-da packing arrangement. This view is similar to that shown in Figure 3B, but the helices have been moved closer together and overlapped to emphasize the layers and packing interactions. Residues at overlapping a and d positions contribute to intrasubunit (dark gray) or intersubunit (light gray) packing interactions; residues at the x positions (black) stabilize both the intra- and intersubunit interface, as summarized in Fig. 3B and 5A. B)
B) Helical wheel cross-section of a possible a-d bundle. This HAMP packing arrangement has not been observed experimentally, but was suggested to be the functional alternative to the x-da signaling state (Hulko et al., 2006). Overlapping sites indicate packing layers and residue interactions. As in the x-da arrangement, interactions between dark gray a/d residue positions stabilize the intrasubunit interface. However, the black a/d residue positions that stabilize the intersubunit interface are x residues in the x-da arrangement of (A). The e (AS1) and g (AS2) positions (light gray shading) correspond to the noncritical a (AS1) and d (AS2) residues in the x-da arrangement.
C) Summary of null (Tsr−) lesions in the AS1 and AS2 helices. Wild-type amino acids and residue numbers are given below the AS1 and AS2 cartoons. Shading of the residue positions corresponds to that of the x, d, and a positions in the HAMP bundles of (A) and (B). Boxes enclose amino acid replacements that caused moderate to severe jamming behavior (E2 or E3 classification, see Table S1). Residue interactions in the four packing layers are shown at the bottom of the figure.