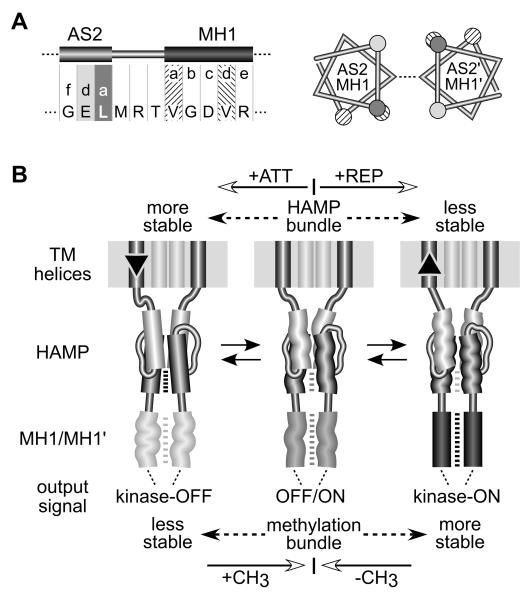
Fig. 8. Mechanistic model of HAMP signaling.
A) Junction of the AS2 helix and the first methylation helix (MH1) of the kinase control domain. Residue positions for AS2 are shaded as in Figures 3 and 7. The N-terminal (MH1) and C-terminal (MH2) methylation helices form a 4-helix coiled-coil in the Tsr dimer (Kim et al., 1999) (see Figure 1), with packing interactions mediated by a-d heptad repeats (Kim et al., 1999; Alexander & Zhulin, 2007). Left: The heptad packing residues of MH1 are four residues out of phase with those of AS2. Right: Helical wheels of the Tsr subunits at the AS2/MH1 junction, showing the alignment of residues in the AS2 helices as they emerge from a stable x-da bundle. The x positions (not highlighted) are connected by a dashed line. The a and d positions of the MH1 helices are nearly 180° out-of-phase, which should destabilize the methylation helices and packing interactions.
B) A modulated dynamics model of HAMP signaling. The model proposes that the x-da HAMP bundle is important to both the kinase-activating and kinase-deactivating signaling states because it controls the range of inter-subunit motions of the kinase control domain in the Tsr dimer. However, the dynamic states of the x-da bundle and methylation helices are oppositionally coupled through an out-of-register helical phase relationship; see (A). An unstable x-da bundle allows inter-subunit interactions of the methylation helices. Conversely, a stable x-da bundle destabilizes the methylation helix bundle. The structural interactions between AS2 and MH1 helices are bi-directional. Changes in methylation state modulate stability of the methylation region, which in turn impacts stability of the HAMP bundle. Importantly, this model predicts that attractant stimuli suppress CheA activity by enhancing stability of the HAMP bundle, whereas repellent stimuli should destabilize the bundle. These stimulus-induced changes in HAMP stability could be triggered by small vertical displacements of the transmembrane segments (TM2) adjoining the AS1 helices. The TM2/AS1 connection could act like a control cable to adjust structural tension on the HAMP domain, altering its dynamic properties. See text for additional explanation.