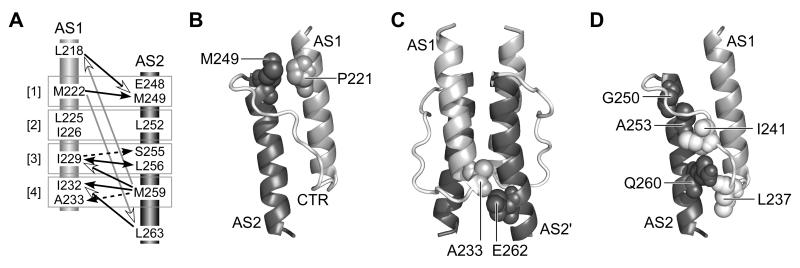
Fig. 9. Structural features of the Tsr-HAMP bundle.
A) Summary of AS1-AS2 suppression effects. Arrows point from the null residue to the suppressor residue. Numbers in brackets identify the packing layers. In the context of the x-da bundle structure, suppression effects that should primarily influence the stability of intrasubunit packing are indicated by black lines and arrowheads; effects that should primarily influence the stability of intersubunit packing are indicated by dashed black lines and arrowheads. Suppression effects involving the bundle-capping residues (L218, L263) that could influence both intra- and intersubunit interactions are indicated by open arrowheads: black lines for adjacent layer interactions and gray lines for long-range interactions.
B) Relative positions of P221 and M249 residues in the modeled Tsr-HAMP structure. Only one subunit is shown; the other subunit would be behind these helices.
C) Relative positions of A233 and E262 residues in the modeled Tsr-HAMP structure. The residues abut one another at the subunit interface.
D) Relative positions of AS2 and critical connector residues in the modeled Tsr-HAMP structure. One subunit is shown, as in (B). The side-chains of connector residues L237 and I241 (white, space-filled) project into the AS1-AS2 cleft of the same subunit. Amino acid replacements at AS2 residues G250, A253, and Q260 (dark gray, space-filled) may disrupt HAMP function by displacing the critical connector residues.