Figure 2.
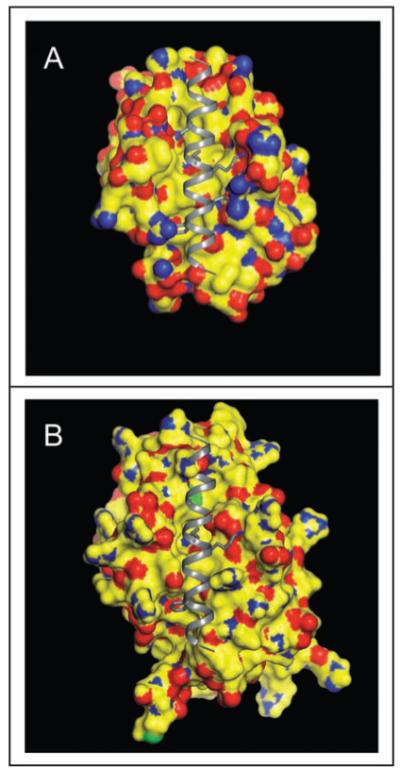
Beclin 1 BH3 domain bound to the hydrophobic surface groove of Bcl-XL (A) or Bcl-2 (B). The molecular surface of Bcl-2/Bcl-XL is color-coded by atom type: yellow represents carbon, blue represents nitrogen, red represents oxygen, and green represents sulfur. The Beclin 1 BH3 helix is shown in gray ribbon. Conserved residues within the BH3 helix, L112, L116, D121 and F123, that are involved in binding to the hydrophophic groove of Bcl-2 homologs are represented in atomic detail with atoms colored as above, except that carbon is depicted in grey. This figure demonstrates that conformational changes are required to accommodate a BH3 domain in the hydrophobic grooves of Bcl-2/Bcl-XL. The crystallographic structure of the Beclin 1 BH3 domain bound to Bcl-XL has been previously determined41 whereas in (B), the Beclin 1 BH3 domain is modeled into a molecular surface representation of Bcl-2, based on a structural superposition of Bcl-2 and Bcl-XL.
