Abstract
Background and Aims
Boron (B) toxicity triggers the formation of reactive oxygen species in plant tissues. However, there is still a lack of knowledge as to how B toxicity affects the plant antioxidant defence system. It has been suggested that ascorbate could be important against B stress, although existing information is limited in this respect. The objective of this study was to analyse how ascorbate and some other components of the antioxidant network respond to B toxicity.
Methods
Two tomato (Solanum lycopersicum) cultivars (‘Kosaco’ and ‘Josefina’) were subjected to 0·05 (control), 0·5 and 2 mm B. The following were studied in leaves: dry weight; relative leaf growth rate; total and free B; H2O2; malondialdehyde; ascorbate; glutathione; sugars; total non-enzymatic antioxidant activity, and the activity of superoxide dismutase, catalase, ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, ascorbate oxidase and l-galactose dehydrogenase.
Key Results
The B-toxicity treatments diminished growth and boosted the amount of B, malondialdehyde and H2O2 in the leaves of the two cultivars, these trends being more pronounced in ‘Josefina’ than in ‘Kosaco’. B toxicity increased ascorbate concentration in both cultivars and increased glutathione only in ‘Kosaco’. Activities of antioxidant- and ascorbate-metabolizing enzymes were also induced.
Conclusions
High B concentration in the culture medium provokes oxidative damage in tomato leaves and induces a general increase in antioxidant enzyme activity. In particular, B toxicity increased ascorbate pool size. It also increased the activity of l-galactose dehydrogenase, an enzyme involved in ascorbate biosynthesis, and the activity of enzymes of the Halliwell–Asada cycle. This work therefore provides a starting point towards a better understanding of the role of ascorbate in the plant response against B stress.
Key words: Boron toxicity, lipid peroxidation, antioxidant enzymes, ascorbate, glutathione, tomato
INTRODUCTION
Boron (B) toxicity, an important agricultural problem that limits crop productivity in different regions of the world, can occur in B-rich soils or in soils exposed to B-rich irrigation waters, fertilizers, sewage sludge, or fly ash (Nable et al., 1997). Furthermore, in recent years, B toxicity has attracted increasing interest owing to the greater demand for desalinated water, in which the B concentration may be too high for healthy irrigation (Parks and Edwards, 2005).
The typical symptoms shown by plants exposed to excess B are reduced vigour, delayed development, leaf burn (chlorotic and necrotic patches in older leaves), and decreased number, size and weight of fruits (Paull et al., 1992; Nable et al., 1997). However, despite the importance of this nutritional disorder, it is not understood why B is toxic to plants, or how tolerant plants avoid toxicity (Reid et al., 2004). It has long been known that the optimum B level for one species could be either toxic or insufficient for other species (Blevins and Lukaszewski, 1998). Genetic variation in response to high B concentrations has prompted investigation into the mechanism operating in plants against B excess. In wheat (Triticum aestivum) and barley (Hordeum vulgare) cultivars, several possible tolerance mechanisms have been proposed, these operating mainly by exclusion (Paull et al., 1992; Hayes and Reid, 2004). These mechanisms are based on studies demonstrating an ability of plants to accumulate less B in shoots, although the transporters which participate in the exclusion process have not yet been identified. On the other hand, it has been suggested that an antioxidant response may reduce B-toxicity damage in some plants (Gunes et al., 2006). This antioxidant response is considered to be a critical process for protecting plants against oxidative damage in combination with a wide range of environmental factors (Inzé and Van Montagu, 1995), including UV-light excess, salinity, drought, heavy metals, chilling, oxygen shortage and nutritional deprivation (Mittler, 2002). During oxidative stress, the excess production of reactive oxygen species (ROS) causes membrane damage that eventually leads to cell death. For protection against ROS, plants contain antioxidant enzymes such as superoxide dismutase (SOD) (EC 1·15·1·1), catalase (CAT) (EC 1·11·1·6) or ascorbate peroxidase (APX) (EC 1·11·1·11), as well as a wide array of non-enzymatic antioxidants (Mittler, 2002; Blokhina et al., 2003).
As in most ionic stresses, toxic levels of B cause the formation of ROS. Karabal et al. (2003) observed in barley cultivars that B toxicity induced oxidative and membrane damage in leaves. Recently, in apple (Malus domestica) and grapevine (Vitis vinifera), it has been reported that B toxicity induces oxidative damage by lipid peroxidation and hydrogen peroxide accumulation (Molassiotis et al., 2006; Gunes et al., 2006). Regarding the relationship between non-enzymatic antioxidants and B toxicity, little information is available. Studying apple rootstock, Molassiotis et al. (2006) and Sotiropoulos et al. (2006) found that the non-enzymatic antioxidant activity increased with increasing B concentrations in the culture medium. Nevertheless, it has been shown that B excess inhibited the formation of glutathione in sunflower (Helianthus annuus) leaves (Ruiz et al., 2003) and tocopherol in orange (Citrus sinensis) plants (Keles et al., 2004). On the other hand, in recent years a close relationship has been discerned between B and ascorbate metabolism (Blevins and Lukaszewski, 1998; Brown et al., 2002). Ascorbate or vitamin C is the most abundant antioxidant in plants, found in all subcellular compartments including the apoplast. It has been suggested that plants with increased ascorbate concentration may improve their growth and stress resistance (Smirnoff, 2000), even under B excess (Keles et al., 2004). In addition, ascorbate regeneration by the Halliwell–Asada cycle is a key antioxidant mechanism against ROS formation (Mittler, 2002). However, more data on the response of the plant's antioxidant defence system to B toxicity are required.
In view of the above, the present study was designed to investigate the oxidative damage, antioxidant system and ascorbate pool of two tomato (Solanum lycopersicum) cultivars subjected to high B levels in the root medium. The results show that B toxicity causes oxidative damage in tomato leaves and that antioxidant defences, particularly related to ascorbate, are increased.
MATERIALS AND METHODS
Plant material and growth conditions
The two cultivars of tomato plants [Solanum lycopersicum L. (Lycopersicon esculentum Mill.)] used in the present study were ‘Josefina’ and ‘Kosaco’. Seeds of the two cultivars were germinated and grown for 30 d in cell flats (cell size, 3 × 3 × 10 cm) filled with a peatlite mixture, and the flats were placed on benches in an experimental greenhouse in southern Spain (Granada, Saliplant S.L.). The 30-d-old seedlings were transferred to a controlled environmental chamber used with relative humidity of 60–80 %, temperature 25 °C/15 °C (day/night), and 16-h/8-h photoperiod at a PPFD (photosynthetic photon-flux density) of 350 µmol m−2 s−1 (measured at the top of the plants with a 190 SB quantum sensor, LI-COR Inc., Lincoln, NE, USA). Plants were grown in individual pots (25 cm upper diameter, 17 cm lower diameter, 25 cm in height) of 8 L volume, filled with vermiculite. Throughout the experiment, the plants were grown in a nutrient solution containing: 4 mm KNO3, 3 mm Ca(NO3)2, 2 mm MgSO4, 1 mm KH2PO4, 1 mm NaH2PO4, 2 µm MnCl2, 1 µm ZnSO4, 0·25 µm CuSO4, 0·1 µm Na2MoO4, 5 µm Fe-EDDHA and 50 µm H3BO3. The nutrient solution (pH 5·8) was renewed every 3 d and the vermiculite partly rinsed with Millipore-filtered water in order to avoid nutrient accumulation.
The B treatments (0·50 and 2·00 mm H3BO3) were started 42 d after sowing and maintained for 14 d, when most of the ‘Josefina’ plants showed clear symptoms of B toxicity. The control treatment received the complete nutrient solution alone (0·05 mm B). The experimental design was a randomized complete block with three treatments, arranged in individual pots with six plants per treatment, and three replicates. The experiment was repeated three times under the same conditions (n = 9).
Plant sampling and determination of relative leaf growth rate
To determine the relative leaf growth rate (RGRL), leaves from three plants per cultivar were sampled on day 42 after sowing, immediately before the start of the B treatment (Ti). The leaves were dried in a forced-air oven at 70 °C for 24 h, and dry weight (DW) was recorded as grams per plant. The remaining plants were sampled 56 d after sowing (day 14 of treatments, TF). Leaves were rinsed three times in distilled water after cleaning with non-ionic detergent at 1 %, and then blotted on filter paper. From each treatment, half the leaves were used fresh for analysis of free B, malondialdehyde (MDA), H2O2, enzymatic activity, sugars, glutathione, ascorbate and total non-enzymatic antioxidant activity. The other half was used to determine the DW of leaves and total B concentration. The relative growth rate was calculated from the increase in leaf DW at the beginning and at the end of the B treatment, using the equation RGRL = (ln DWF – ln DWi)/(TF – Ti) where T is the time and the subscripts denote the first and last sampling (i.e. days 0 and 14, respectively, after the B treatment; Bellaloui and Brown, 1998).
Boron analysis
The total B concentration was analysed after digestion of 0·15 g dry and milled leaf material with H2SO4 (5 mL at 98 %) and H2O2 (30 %). For free B extraction, a batch of 0·15 g of fresh leaves cut into discs 7 mm in diameter were placed in a test tube with 10 mL infiltration medium (propanol 1 %, which increases membrane permeability), then subjected to vacuum for 25 min, and later filtered. To measure B concentration, the azomethine-H method was followed and the absorbance was read by spectrophotometry at 410 nm (Wolf, 1974). The total B concentration was expressed as μg g−1 DW and free B as μg g−1 fresh weight (FW).
Concentration of MDA and H2O2
For the MDA assay, leaves were homogenized with 5 mL of 50 mm solution containing 0·07 % NaH2PO4.2H2O and 1·6 % Na2HPO4 · 12H2O, ground with a mortar and pestle, and centrifuged at 20 000 g for 25 min in a refrigerated centrifuge. For measurement of MDA concentration, 4 mL of 20 % trichloroacetic acid containing 0·5 % thiobarbituric acid was added to a 1-mL aliquot of the supernatant. The mixture was heated at 95 °C for 30 min and then quickly cooled in an ice bath. After the tube was centrifuged at 10 000 g for 10 min, the absorbance of the supernatant was read at 532 nm. The value for the non-specific absorption at 600 nm was subtracted from the reading at 532 nm. The concentration of MDA was calculated using the MDA extinction coefficient of 155 mm−1 cm−1 (Heath and Packer, 1968; Fu and Huang, 2001).
The H2O2 content of leaf samples was colorimetrically measured as described by Mukherjee and Choudhuri (1983). Leaf samples were extracted with cold acetone to determine the H2O2 levels. An aliquot (1 mL) of the extracted solution was mixed with 200 µL of 0·1 % titanium dioxide in 20 % (v/v) H2SO4 and the mixture was then centrifuged at 6000 g for 15 min. The intensity of yellow colour of the supernatant was measured at 415 nm. The H2O2 concentration was calculated from a standard curve plotted within the range of 100–1000 nmol H2O2.
Non-enzymatic antioxidant activity, ascorbate and glutathione
Total non-enzymatic antioxidant activity was measured using the Ferric Reducing Ability of Plasma (FRAP) and Trolox Equivalent Antioxidant Capacity (TEAC) assays. For both tests, the leaf tissue (0·5 g) was homogenized in 5 mL methanol. The homogenate was filtered and centrifuged at 12 800 × g for 2 min. The FRAP assay was made with FRAP reagent, i.e. 1 mm 2,4,6-tripyridyl-2-triazine (TPTZ) and 20 mm ferric chloride in 0·25 m sodium acetate (pH 3·6). An aliquot of 100 µL of leaf extract (0·5 g/10 mL in methanol) was added to 2 mL of FRAP reagent and thoroughly mixed. After the mixture was left at 20° C for 5 min, absorbance at 593 nm was measured. Calibration was against a standard curve (25–1600 µm ferrous iron) using freshly prepared ammonium ferrous sulfate (Benzie and Strain, 1996; Rosales et al., 2006).
The TEAC assay was performed according to a modified version of the method of Re et al. (1999) and Cai et al. (2004). ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation (ABTS•+) was produced with 7 mm ABTS and 2·45 mm potassium persulfate after incubation at room temperature in darkness for 16 h. After incubation, the ABTS•+ solution was diluted with methanol to an absorbance of 0·70 ± 0·02 at 734 nm. An aliquot of 100 µL of leaf extract (0·5 g/10 mL in methanol) was added to 3·9 mL of diluted ABTS•+ solution and mixed thoroughly. The reactive mixture was allowed to stand at room temperature for 6 min and the absorbance was immediately recorded at 734 nm. Trolox standard solution (0–15 µm) in methanol was prepared and assayed under the same conditions. The absorbance of the resulting oxidized solution was compared with that of the calibrated Trolox standard. Results were expressed in terms of Trolox-equivalent antioxidant capacity (μmol Trolox equivalents per g FW).
Reduced ascorbate (ASA), dehydroascorbate (DHA) and total ascorbate (ASA + DHA) were determined spectrophotometrically following Gossett et al. (1994). The amounts of reduced ascorbate and total ascorbate were assayed from the same extract. ASA standards ranging between 0·1 and 1·5 µm l-ascorbic acid in metaphosphoric acid were analysed in the same manner as the extracts. For each sample, DHA was estimated from the difference between total ascorbate and ASA.
Reduced glutathione (GSH), oxidized glutathione (GSSG) and total glutathione (GSH + GSSG) were determined following Gossett et al. (1994). From the same extract, GSSG and total glutathione were assayed. A standard curve was developed by preparing solutions of 0·002–0·0001 g mL−1 GSH in 60 mL metaphosphoric acid (pH 2·8) containing 1 mm EDTA, diluting 1 : 2000 with 50 mL L−1 Na2PO4, and analysing in the same manner as for the extracts. Levels of GSH were estimated as the difference between total glutathione and GSSG.
Activity of antioxidant enzymes
SOD (EC 1·15·1·1) activity was assayed by monitoring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT), according to the methods of Giannopolitis and Ries (1977) and Beyer and Fridovitch (1987), with some modifications (Yu et al., 1998). A 5-mL reaction mixture was used, containing 50 mm Na2CO3 (pH 10·0), 13 mm methionine, 0·025 % (v/v) Triton X-100, 63 µm NBT, 1·3 µm riboflavin and an appropriate aliquot of enzyme extract. The reaction mixtures were illuminated for 15 min at a PPFD of 380 µmol−2 s−1. Mixtures not illuminated were used to correct for background absorbance. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of the reduction of NBT as monitored at 560 nm.
CAT (1·11·1·6) activity was determined by following the consumption of H2O2 at 240 nm for 5 min (Nakano and Asada, 1981; Rao et al., 1997). The reaction mixture (3 mL total volume) contained 25 mm Tris-acetate buffer (pH 7·0), 0·8 mm Na-EDTA and 20 mm H2O2, and enzyme assay was performed at 25 °C.
The enzymes APX (EC 1·11·1·11) and glutathione reductase (GR; EC 1·6·4·1) were assayed following Rao et al. (1996). APX activity was determined by registering the absorbance change at 290 nm for 3 min of a reaction mixture (3·75 mL) containing 100 mm phosphate potassium buffer (pH 7·5), 0·5 mm ASA, 0·2 mm H2O2 and 0·75 mL enzyme extract. GR activity was measured after monitoring the oxidation of NADPH at 340 nm for 3 min in a reaction mixture (3·5 mL) containing 100 mm Tris-HCl (pH 7·8), 2 mm Na2-EDTA, 0·2 mm NADPH, 0·5 mm GSSG and 0·75 mL enzyme extract.
Dehydroascorbate reductase activity (DHAR; EC 1·8·5·1) was measured at 265 nm for 3 min following the change in absorbance resulting from the formation of ASA (Nakano and Asada, 1981). The reaction mixture (3·1 mL) contained 25 mm phosphate sodium buffer (pH 7), 2·5 mm GSH, 0·4 mm DHA and 0·1 mL enzyme extract. The molar extinction coefficient used to calculate the activity of this enzyme was 7·0 mm−1 cm−1. In addition, the enzyme monodehydroascorbate reductase (MDHAR; EC 1·6·5·4) was assayed by registering the change in absorbance of the samples at a wavelength of 340 nm (Foyer et al., 1989). The reaction mixture (3·3 mL) contained 100 mm HEPES-HCl (pH 7·6) buffer, 2·5 mm ASA, 25 mm NADPH and 300 µL enzyme extract. The NADPH molar extinction coefficient of 6·22 mm−1 cm−1 was used to calculate the MDHAR activity.
Ascorbate oxidase (AO; EC 1·10·3·3) activity was measured according to a modified version of the method of García-Pineda et al. (2004), based on the fact that ASA absorbs at 265 nm whereas the oxidation product, MDHA, does not. The reaction mixture (1 mL) consisted of 0·025 mm citrate/phosphate buffer (pH 5·6), 0·08 mm l-ascorbic acid, 0·02 mm neutralized Na2-EDTA, 0·15 g L−1 bovine serum albumin solution, and up to 200 µL of enzyme extract (extinction coefficient = 9246 m−1 cm−1 at 265 nm).
Finally, l-galactose dehydrogenase activity (l-GalDH) was determined following the method of Gatzek et al. (2002). For this, a reaction mixture (1·1 mL) containing 50 mm Tris-HCl (pH 7·5) buffer, 0·1 mm NAD, 5 mm l-galactose and 0·1 mL of plant extract was used. The formation of NADH during the reaction was measured at 340 nm and l-GalDH activity was calculated according to the molar extinction coefficient of 6·22 mm−1 cm−1.
The protein concentration of the extracts was determined according to the method of Bradford (1976), using bovine serum albumin as standard.
Determination of sugar concentrations
Sucrose, glucose and fructose were extracted and quantified by use of a kit (Roche Biopharm, France), based on enzyme-linked formation of NADPH.
Statistical analysis
The data compiled were submitted to an analysis of variance (ANOVA) and the differences between the means were compared by Duncan's multiple-range test (P< 0·05). In addition, to ascertain whether the type of cultivar used in the experiment significantly influenced the results, a two-way ANOVA was used and the means were compared by Fisher's least-significant difference test (LSD).
RESULTS AND DISCUSSION
Tomato leaf biomass and RGRL decreased as a result of higher B concentration in the root medium (Table 1). Both cultivars also showed an increase in the total and free B concentration, the latter being higher in ‘Josefina’ than in ‘Kosaco’ (Table 2). A reduction in growth and increase of B concentration in the plant tissues as a consequence of B toxicity has previously been observed in tomato (Gunes et al., 1999), sunflower (Ruiz et al., 2003) and barley (Karabal et al., 2003).
Table 1.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on leaf dry weight and relative leaf growth rate (RGRL) of two tomato cultivars (‘Kosaco’ and ‘Josefina’)
| Dry weight (g) | RGRL (g g−1 DW d−1) | |||
|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 1·85 ± 0·06a† | 2·16 ± 0·06a | 0·108 ± 0·002a | 0·108 ± 0·002a |
| 0·50 mm | 1·84 ± 0·05a | 1·84 ± 0·06b | 0·108 ± 0·003a | 0·097 ± 0·002b |
| 2·00 mm | 1·46 ± 0·05b | 1·62 ± 0·05c | 0·092 ± 0·001b | 0·088 ± 0·001c |
| P | **§ | ** | ** | *** |
| ‘Kosaco’ | 1·72b‡ | 0·103a | ||
| ‘Josefina’ | 1·87a | 0·098b | ||
| P | * | * | ||
| LSD0·05 | 0·11 | 0·004 | ||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by *P < 0·05, **P < 0·01, ***P < 0·001.
Table 2.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on total and free B concentration in leaves of two tomato cultivars (‘Kosaco’ and ‘Josefina’)
| Total B (µg g−1 DW) | Free B (µg g−1 FW) | |||
|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 87·71 ± 3·57b† | 94·92 ± 5·63c | 10·03 ± 0·81b | 13·02 ± 3·63b |
| 0·50 mm | 209·18 ± 43·37b | 367·18 ± 3·61b | 15·14 ± 1·38b | 22·45 ± 0·29b |
| 2·00 mm | 627·63 ± 43·61a | 1055·58 ± 18·25a | 64·55 ± 4·70a | 103·85 ± 9·83a |
| P | ***§ | *** | *** | *** |
| ‘Kosaco’ | 308·17b‡ | 29·91b | ||
| ‘Josefina’ | 505·90a | 46·44a | ||
| P | ** | * | ||
| LSD0·05 | 108·54 | 12·04 | ||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by *P < 0·05, **P < 0·01, ***P < 0·001.
The MDA and H2O2 concentrations were measured in leaves as an indicator of oxidative stress (Mittler, 2002). Both parameters increased significantly under B toxicity (Fig. 1). Mittler (2002) proposed that membrane damage might be caused by high H2O2 levels, which could accelerate the Haber–Weiss reaction, resulting in hydroxyl radical (OH•–) formation and thus lipid peroxidation. However, Karabal et al. (2003) found no relationship between the H2O2 concentration and lipid peroxidation in barley subjected to high B levels. In accordance with the present results, others have recently found that excess B increased both MDA and H2O2 concentrations in apple rootstock (Molassiotis et al., 2006) and grape (Gunes et al., 2006).
Fig. 1.
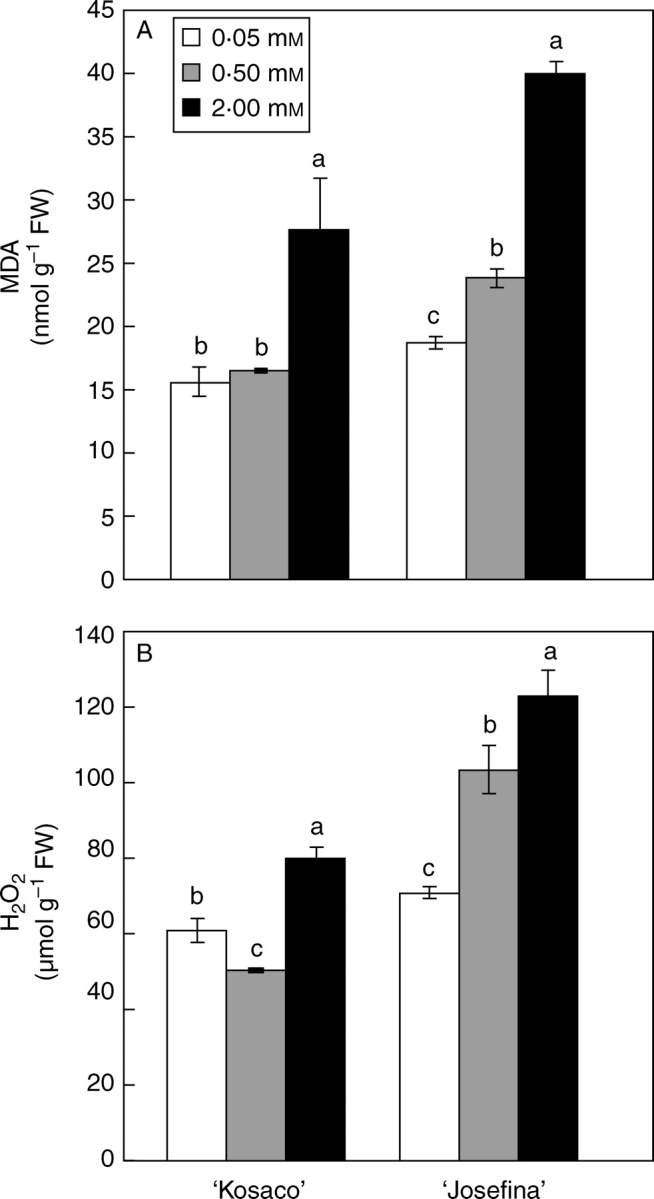
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on (A) MDA and (B) H2O2 concentration in leaves of two tomato cultivars: ‘Kosaco’ and ‘Josefina’. Bars represent means ± s.e. (n = 9); for each cultivar, bars followed by the same letters are not significantly different using Duncan's test (P = 0·05).
Non-enzymatic antioxidant activity is represented by a series of antioxidant molecules that the plant uses against ROS formation (Mittler, 2002). FRAP and TEAC were used to provide information on total non-enzymatic antioxidant activity. Except in the FRAP test made on ‘Josefina’, the assays showed significantly greater antioxidant activity under the treatment with 2 mm B (Table 3). Similarly, in apple Molassiotis et al. (2006) and Sotiropoulos et al. (2006) found that B toxicity increased non-enzymatic antioxidant activity.
Table 3.
FRAP and TEAC assay in leaves of two tomato cultivars (‘Kosaco’ and ‘Josefina’) subjected to 0·05 mm B (control) and B toxicity (0·5 and 2 mm)
| FRAP (µmol g−1 FW) | TEAC (µmol g−1 FW) | |||
|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 1005·13 ± 5·18b† | 729·62 ± 0·90b | 6·20 ± 0·07b | 5·01 ± 0·09b |
| 0·50 mm | 955·62 ± 4·71c | 769·33 ± 12·16a | 6·02 ± 0·04b | 5·29 ± 0·10b |
| 2·00 mm | 1025·41 ± 4·54a | 721·28 ± 8·94b | 6·82 ± 0·06a | 6·66 ± 0·06a |
| P | ***§ | * | *** | *** |
| ‘Kosaco’ | 995·39a‡ | 6·34a | ||
| ‘Josefina’ | 737·41b | 5·65b | ||
| P | *** | *** | ||
| LSD0·05 | 29·01 | 0·27 | ||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by *P < 0·05, **P < 0·01, ***P < 0·001.
To investigate this latter aspect further, leaf ascorbate and glutathione concentrations were also determined. These antioxidant molecules are found in high concentrations in chloroplasts and other cellular compartments and have a major defence function against oxidative stress (Noctor and Foyer, 1998). In the present experiment, the ascorbate concentration increased significantly in both cultivars (Table 4). Boron application is known to elevate the ascorbate concentration in plant tissues as compared with B-deficient conditions (Blevins and Lukaszewski, 1998). Increased ascorbate concentration by foliar applications of B was reported in potato (Solanum tuberosum; Mondy and Munshi, 1993). More recently, Keles et al. (2004), studying orange, observed a significant rise in this antioxidant under conditions of excess B in the medium, proposing that this could be important against ROS formation. In the current study, with 0·5 and 2 mm B, reduced ascorbate increased, respectively, by 64 and 91 % in ‘Kosaco’ and by 13 and 72 % in ‘Josefina’ (Table 4). Given that ascorbate can directly scavenge superoxide, hydroxyl radicals and singlet oxygen and reduce H2O2 to water via the APX reaction (Noctor and Foyer, 1998), the increase of ascorbate concentration in tomato plants suggests an important role for this antioxidant against oxidative stress provoked by B toxicity. Moreover, with 0·5 and 2 mm B, the DHA concentration was elevated, respectively, by 230 and 1561 % in ‘Kosaco’ and by 84 and 134 % in ‘Josefina’, resulting in a generally more oxidized ascorbate pool (Table 4). This suggests that oxidative stress caused by B toxicity both increases the ascorbate pool and increases its oxidation. Regarding glutathione, a significant increase was observed only in ‘Kosaco’ (Table 5). As with results under high-salinity conditions (Ruiz and Blumwald, 2002), this antioxidant could be important in protecting against oxidative stress triggered by high B concentrations. In sunflower, Ruiz et al. (2003) showed that B toxicity inhibited glutathione synthesis and that the external application of this antioxidant reduced phytotoxicity caused by the high B concentration.
Table 4.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on ASA, DHA, total ascorbate concentration and status redox (ASA/Total ascorbate) in leaves of two tomato cultivars (‘Kosaco’ and ‘Josefina’)
| ASA (nmol g−1 FW) | DHA (nmol g−1 FW) | Total ascorbate (nmol g−1 FW) | ASA/Total ascorbate (%) | |||||
|---|---|---|---|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 198·94 ± 0·33c† | 150·14 ± 3·13c | 32·29 ± 0·35c | 47·03 ± 3·13c | 231·23 ± 14·11c | 197·18 ± 9·40c | 86·01 ± 0·57a | 76·10 ± 1·73a |
| 0·50 mm | 326·69 ± 16·40b | 169·63 ± 6·39b | 106·53 ± 16·40b | 86·66 ± 6·38b | 433·21 ± 18·45b | 256·28 ± 9·58b | 75·33 ± 3·75b | 66·33 ± 2·60b |
| 2·00 mM | 379·95 ± 14·35a | 258·68 ± 1·06a | 536·19 ± 14·35a | 112·16 ± 1·04a | 916·14 ± 6·15a | 370·84 ± 9·40a | 41·32 ± 1·45c | 69·66 ± 3·33b |
| P | ***§ | *** | *** | *** | *** | *** | *** | * |
| ‘Kosaco’ | 301·86a‡ | 225·00a | 526·86a | 67·55a | ||||
| ‘Josefina’ | 192·82b | 81·95b | 274·77b | 70·66a | ||||
| P | *** | * | *** | ns | ||||
| LSD0·05 | 29·95 | 115·18 | 124·95 | 10·77 | ||||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by *P < 0·05, ***P < 0·001 and ns (not significant, P > 0·05).
Table 5.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on GSH, GSSG and total glutathione concentration in leaves of two tomato cultivars (‘Kosaco’ and ‘Josefina’)
| GSH (nmol g−1 FW) | GSSG (nmol g−1 FW) | Total glutathione (nmol g−1 FW) | ||||
|---|---|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 470·64 ± 4·84c† | 452·00 ± 2·61a | 166·06 ± 4·83b | 174·75 ± 2·61a | 636·03 ± 0·40b | 626·75 ± 11·49a |
| 0·50 mm | 918·03 ± 4·51a | 460·72 ± 6·73a | 105·82 ± 4·51c | 154·35 ± 6·73b | 1023·85 ± 45·74a | 615·07 ± 34·31a |
| 2·00 mm | 663·65 ± 2·76b | 468·86 ± 0·71a | 195·80 ± 2·76a | 177·93 ± 0·71a | 859·45 ± 88·28a | 646·79 ± 13·77a |
| P | ***§ | ns | *** | * | ** | ns |
| ‘Kosaco’ | 684·11a‡ | 155·89a | 840·11a | |||
| ‘Josefina’ | 460·53b | 169·01a | 629·54b | |||
| P | *** | ns | ** | |||
| LSD0·05 | 103·54 | 17·04 | 116·99 | |||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by *P < 0·05, **P < 0·01, ***P < 0·001 and ns (not significant, P > 0·05).
To study the enzymatic response of the tomato plants against B toxicity, antioxidant enzymes in the leaves were analysed, such as SOD, CAT and APX, in addition to other enzymes that participate in the regeneration of ascorbate in the Halliwell–Asada cycle. With 2 mm B, CAT and APX activities increased significantly in both cultivars with respect to the control treatment (Fig. 2B, C), while SOD activity increased with 2 mm B only in ‘Kosaco’ (Fig. 2A). SOD activity increased significantly with 0·5 mm in both cultivars (Fig. 2A). Boron toxicity effects on enzyme activities in leaves vary widely, depending on the plant species. In barley, Karabal et al. (2003) found that excess B in the medium significantly increased APX activity but not that of SOD. Recently, in apple rootstock, it was reported that an increase of B concentration in the culture medium from 0·1 to 6 mm reduced CAT activity, while inducing SOD and peroxidase activities in leaves (Molassiotis et al., 2006). However, in grape, Gunes et al. (2006) have reported that B toxicity augments CAT and SOD activity at the same time as it diminishes that of APX.
Fig. 2.
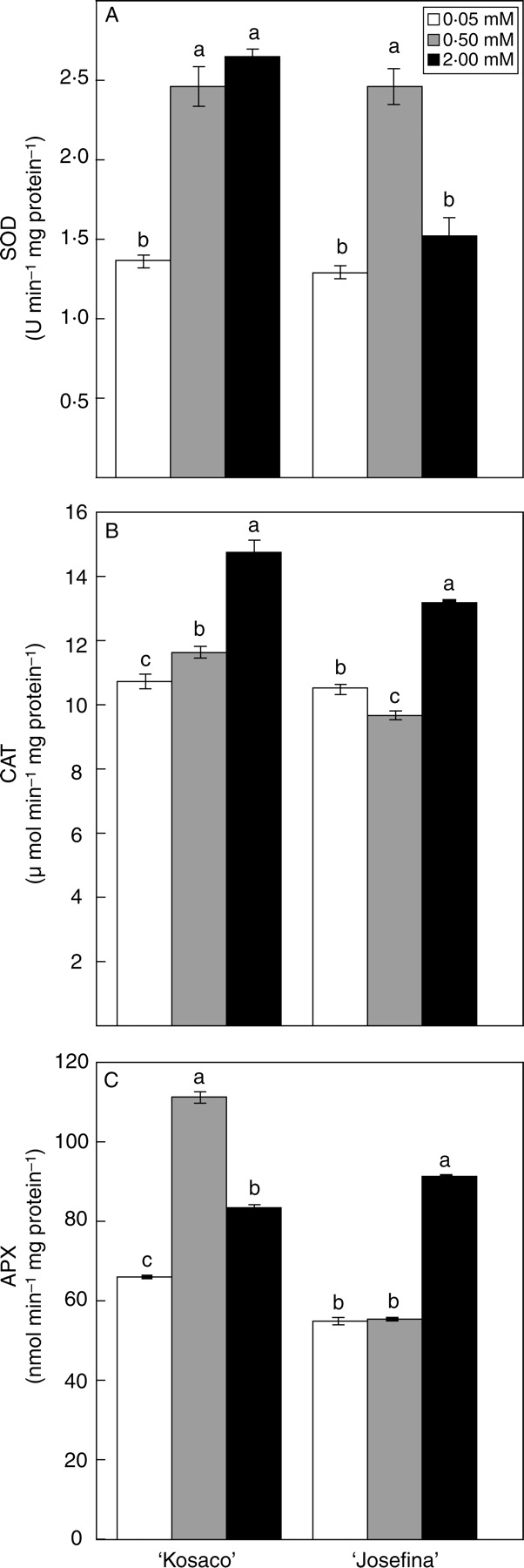
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on (A) SOD, (B) CAT and (C) APX activity in leaves of two tomato cultivars: ‘Kosaco’ and ‘Josefina’. Bars represent means ± s.e. (n = 9); for each cultivar, bars followed by the same letters are not significantly different using Duncan's test (P = 0·05).
The regeneration of ascorbate is also a process of great importance in the antioxidant response. This process involves the enzymes MDHAR, DHAR and GR, which are situated in most cellular compartments (Ishikawa et al., 2006). In the present study the activity of these enzymes increased significantly with 2 mm B in both cultivars (Fig. 3). Recently, it has been suggested that over-expression of MDHAR might both affect ascorbate accumulation and raise the redox status of ascorbate towards reduction, because MDHAR functions upstream of DHAR in the ascorbate recycling pathway (Ishikawa et al., 2006). We found that with 0·5 and 2 mm B, MDHAR increased more than 50 % in both cultivars (Fig. 3). By contrast, both MDHAR and DHAR may still be a limiting factors for the accumulation of reduced ascorbate in leaves, as the redox status decreased with B toxicity in both cultivars (Table 4). There are only a few previous studies that describe the effect of B toxicity on the enzymes of the Halliwell–Asada cycle. In loquat (Eriobotrya japonica), grown under high salinity, an increase of B concentration from 0·01 to 0·2 mm in the culture medium significantly inhibited MDHAR activity but stimulated that of DHAR (López-Gómez et al., 2007), indicating that B can affect the activity of these enzymes under stress conditions. Similar to the results here, B stress increased GR activity in barley (Karabal et al., 2003).
Fig. 3.
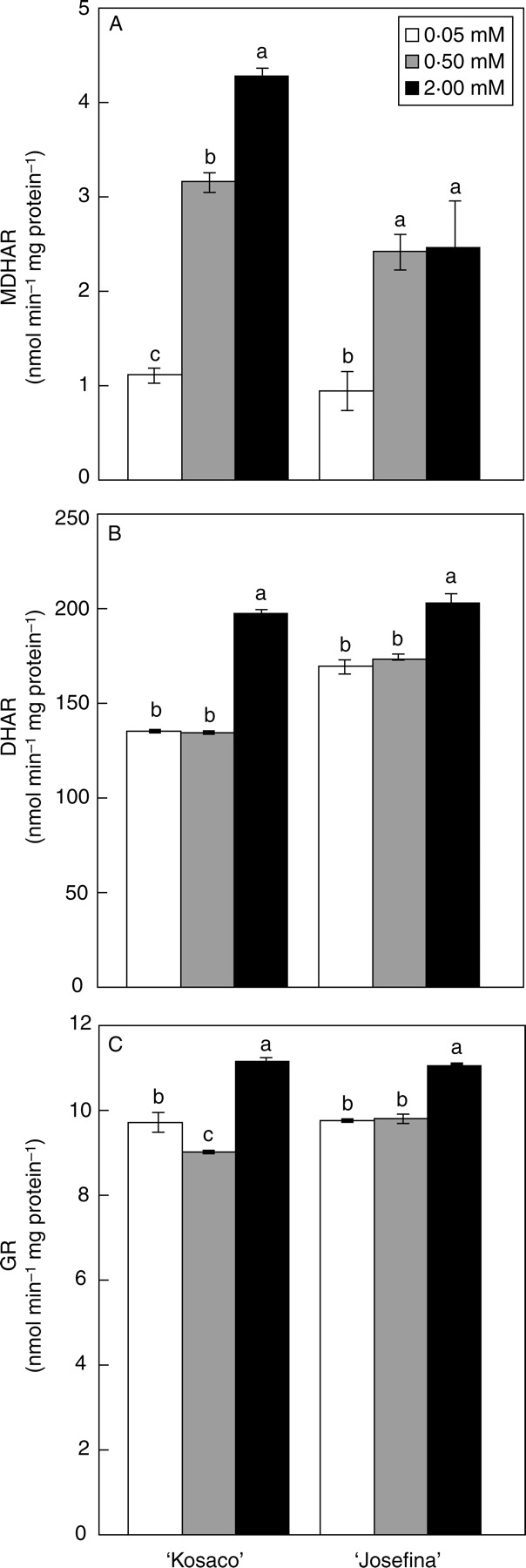
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on (A) MDHAR, (B) DHAR and (C) GR activity in leaves of two tomato cultivars: ‘Kosaco’ and ‘Josefina’. Bars represent means ± s.e. (n = 9); for each cultivar, bars followed by the same letters are not significantly different using Duncan's test (P = 0·05).
In addition, the response of AO activity to B toxicity was measured. This glycoprotein, found in the cell wall, has functions that are still not well understood, although it appears to be linked to the maintenance of the proper redox status in the apoplast and therefore to ASA oxidation to MDHA (Pignocchi et al., 2003). In the current study, the behaviour of the soluble AO fraction was similar in both cultivars, reaching its maximum activity under 0·5 mm B (Table 6). By contrast, when the cell-wall-bound fraction was analysed, a significant increase was found only in ‘Josefina’, although, independently of the treatments, no significant differences were found between the two cultivars (Table 6). Although there are no earlier studies on the effect of B toxicity on AO activity, it has been observed that the activity of this enzyme is induced under other types of stress, for example high temperature or strong solar radiation (Rosales et al., 2006). Recent studies on transgenic plants with altered AO activity indicate that this enzyme could affect adaptation to stress by leading to H2O2 accumulation in the apoplast (Sanmartin et al., 2003; Yamamoto et al., 2005). In the present study, although a certain effect of B toxicity was detected on AO activity, it remains difficult to associate H2O2 levels with the behaviour of this enzyme.
Table 6.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on AO activity in leaves of two tomato cultivars (‘Kosaco’ and ‘Josefina’)
| Free AO (nmol min−1 mg protein−1) | Cell-wall AO (nmol min−1 mg protein−1) | |||
|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 20·69 ± 0·06b† | 17·28 ± 0·26c | 3·54 ± 0·19a | 2·88 ± 0·01b |
| 0·50 mm | 26·68 ± 0·48a | 22·57 ± 0·06a | 2·97 ± 0·01a | 3·40 ± 0·18b |
| 2·00 mm | 14·46 ± 0·29c | 19·00 ± 0·68b | 3·28 ± 0·39a | 5·29 ± 0·43a |
| P | ***§ | *** | ns | ** |
| ‘Kosaco’ | 20·61a‡ | 3·26a | ||
| ‘Josefina’ | 19·62a | 3·86a | ||
| P | ns | ns | ||
| LSD0·05 | 2·33 | 0·75 | ||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by **P < 0·01, ***P < 0·001 and ns (not significant, P > 0·05).
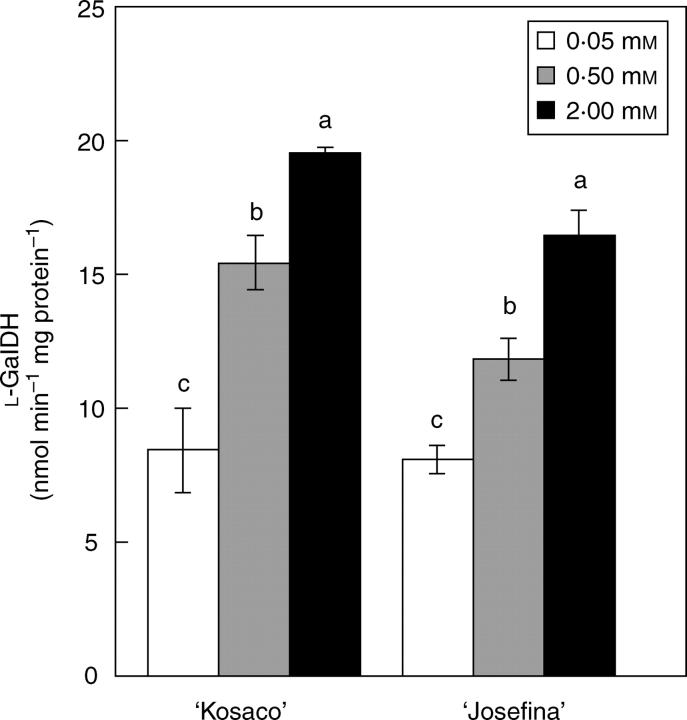
The effect of B toxicity on ascorbate synthesis in plants has not yet been established. In the mannose–galactose pathway, ascorbate is produced from hexoses and by the oxidation of l-galactose, a process involving the enzyme l-GalDH (Ishikawa et al., 2006). In the present experiments, analysis of the glucose, fructose and sucrose concentrations (the early precursors in ascorbate biosynthesis) revealed increases in these sugars under B toxicity without significant differences between ‘Kosaco’ and ‘Josefina’ (Table 7). It has been suggested that the accumulation of soluble carbohydrates in leaves is necessary to alleviate the toxicity symptoms of high B concentrations (Yokota and Konishi, 1990). As under other types of stress, this accumulation of soluble sugars appears to be due to a limitation of their use by some metabolic processes involved in plant growth in favour of others that help to combat oxidative stress, as might be the case with ascorbate synthesis. In agreement with the present study, Keles et al. (2004) reported that in orange plants excess B in the medium boosted the concentration of ascorbate as well as that of glucose and fructose. In terms of l-GalDH activity, the results show that this enzyme increased with the B toxicity treatments in both cultivars (Fig. 4). Increased l-GalDH activity, along with an increased supply of sugar precursor, could indicate a higher capacity for ascorbate synthesis. Furthermore, improved recycling in the Halliwell–Asada cycle could also stabilize and increase the ascorbate pool during B toxicity.
Table 7.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on glucose, fructose and sucrose concentration in leaves of two tomato cultivars (‘Kosaco’ and ‘Josefina’)
| Glucose (mg g−1 FW) | Fructose (mg g−1 FW) | Sucrose (mg g−1 FW) | ||||
|---|---|---|---|---|---|---|
| ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | ‘Kosaco’ | ‘Josefina’ | |
| 0·05 mm | 0·77 ± 0·05b† | 0·27 ± 0·17c | 1·18 ± 0·04c | 0·86 ± 0·17c | 0·81 ± 0·05c | 0·82 ± 0·02c |
| 0·50 mm | 0·69 ± 0·03b | 0·68 ± 0·03b | 1·45 ± 0·01b | 1·39 ± 0·03b | 1·06 ± 0·01b | 1·14 ± 0·01b |
| 2·00 mm | 2·58 ± 0·01a | 2·75 ± 0·01a | 4·46 ± 0·04a | 4·60 ± 0·10a | 1·61 ± 0·01a | 1·33 ± 0·03a |
| P | ***§ | *** | *** | *** | *** | *** |
| ‘Kosaco’ | 1·35a‡ | 2·36a | 1·16a | |||
| ‘Josefina’ | 1·24a | 2·28a | 1·10a | |||
| P | ns | ns | ns | |||
| LSD0·05 | 0·20 | 0·17 | 0·10 | |||
† Values are means ± s.e. (n = 9) and differences between means were compared using Duncan's test (P = 0·05).
‡ Values are means (n = 27) and differences between means were compared using LSD test (P = 0·05).
§ Levels of significance are represented by ***P < 0·001 and ns (not significant, P > 0·05).
Fig. 4.
Effect of 0·05 mm B (control) and B toxicity (0·5 and 2 mm) on l-GalDH activity in leaves of two tomato cultivars: ‘Kosaco’ and ‘Josefina’. Bars represent means ± s.e. (n = 9); for each cultivar, bars followed by the same letters are not significantly different using Duncan's test (P = 0·05).
In conclusion, the present study shows that high B concentrations in the culture medium provoke oxidative damage in leaves of tomato, inducing a general response of antioxidant enzymes. The data also suggest that non-enzymatic antioxidant activity, by synthesizing molecules such as glutathione and ascorbate, could present a protective function against oxidative stress by B. In this sense, B toxicity results in an important effect on the ascorbate pool, on an enzyme involved in ascorbate metabolism (l-GalDH) and on enzymes of the Halliwell–Asada cycle. This work therefore provides a basis for understanding the role of ascorbate and other antioxidants in the response to B toxicity.
ACKNOWLEDGEMENTS
This work was supported by Plan Nacional de I + D + i of Ministerio de Educación y Ciencia, Spain (AGL2006-03164/AGR).
LITERATURE CITED
- Bellaloui N, Brown PH. Cultivar differences in boron uptake and distribution in celery (Apium graveolens), tomato (Lycopersicon esculentum) and wheat (Triticum aestivum) Plant and Soil. 1998;198:153–158. [Google Scholar]
- Benzie IEF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Beyer WF, Fridovitch I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Blevins DG, Lukaszewski KM. Boron in plant structure and function. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:481–500. doi: 10.1146/annurev.arplant.49.1.481. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, et al. Boron in plant biology. Plant Biology. 2002;4:205–223. [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sciences. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Dujardyn M, Lemoine Y. Responses of photosynthesis and the xanthophyll and ascorbate–glutathione cycle to changes in irradiances, photoinhibition and recovery. Plant Physiology and Biochemistry. 1989;27:751–760. [Google Scholar]
- Fu J, Huang B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localizad drought stress. Environmental and Experimental Botany. 2001;45:105–114. doi: 10.1016/s0098-8472(00)00084-8. [DOI] [PubMed] [Google Scholar]
- García-Pineda E, Castro-Mercado E, Lozoya-Gloria E. Gene expression and enzyme activity of pepper (Capsicum annum L.) ascorbate oxidase during elicitor and wounding stress. Plant Science. 2004;166:237–243. [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N. Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant Journal. 2002;30:541–553. doi: 10.1046/j.1365-313x.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutase occurrence in higher plants. Plant Physiology. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett DR, Millhollon EP, Lucas MC. Antioxidants responses to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Science. 1994;34:706–714. [Google Scholar]
- Gunes A, Alpaslan M, Cikili Y, Ozcan H. Effect of zinc on the alleviation of boron toxicity in tomato. Journal of Plant Nutrition. 1999;22:1061–1068. [Google Scholar]
- Gunes A, Soylemezoglu G, Inal A, Bagci EG, Coban S, Sahin O. Antioxidant and stomatal responses of grapevine (Vitis vinifera L.) to boron toxicity. Scientia Horticulturae. 2006;110:279–284. [Google Scholar]
- Hayes JE, Reid RJ. Boron tolerance in barley is mediated by efflux of boron from the roots. Plant Physiology. 2004;136:3376–3382. doi: 10.1104/pp.103.037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Inzé D, Van Montagu M. Oxidative stress in plants. Current Opinion in Biotechnology. 1995;6:153–158. [Google Scholar]
- Ishikawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiologia Plantarum. 2006;126:343–355. [Google Scholar]
- Karabal E, Yücel M, Ökte HA. Antioxidants responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Science. 2003;164:925–933. [Google Scholar]
- Keles Y, Öncel I, Yenice N. Relationship between boron content and antioxidant compounds in Citrus leaves taken from fields with different water source. Plant and Soil. 2004;265:345–353. [Google Scholar]
- López-Gómez E, San Juan MA, Diaz-Vivancos P, Mataix Beneyto J, García-Legaz MF, Hernández JA. Effect of rootstocks grafting and boron on the antioxidant systems and salinity tolerance of loquat plants (Eriobotrya japonica Lindl.) Environmental and Experimental Botany. 2007;60:151–158. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I. Boron induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh) Environmental and Experimental Botany. 2006;56:54–62. [Google Scholar]
- Mondy NI, Munshi CB. Effect of boron on enzymatic discoloration and phenolic and ascorbic acid contents of potatoes. Journal of Agricultural and Food Chemistry. 1993;41:554–558. [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiologia Plantarum. 1983;58:166–170. [Google Scholar]
- Nable OR, Bañuelos GS, Paull JG. Boron toxicity. Plant and Soil. 1997;193:181–198. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- Noctor G, Foyer C. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Parks JL, Edwards M. Boron in the enviroment. Critical Reviews in Environmental Science and Biotechnology. 2005;35:81–114. [Google Scholar]
- Paull JG, Nable RO, Rathjen AJ. Physiological and genetic control of the tolerance of wheat to high concentrations of boron and implications for plant breeding. Plant and Soil. 1992;146:251–260. [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH. The function of ascorbate oxidase in tobacco. Plant Physiology. 2003;132:1631–1641. doi: 10.1104/pp.103.022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP. Ultraviolet-B radiation and ozone-induced biochemical changes in the antioxidant enzymes of Arabidopsis thaliana. Plant Physiology. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes: salicylic acid-mediated oxidative damage requires H2O2. Plant Physiology. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD. A critical analysis of the causes of boron toxicity in plants. Plant Cell and Environment. 2004;25:1405–1414. [Google Scholar]
- Rosales MA, Ruiz JM, Hernández J, Soriano T, Castilla N, Romero L. Antioxidant content and ascorbate metabolism in cherry tomato exocarp in relation to temperature and solar radiation. Journal of the Science of Food and Agriculture. 2006;86:1545–1551. [Google Scholar]
- Ruiz JM, Blumwald E. Salinity-induced glutathione synthesis in. Brassica napus. Planta. 2002;214:965–969. doi: 10.1007/s00425-002-0748-y. [DOI] [PubMed] [Google Scholar]
- Ruiz JM, Rivero RM, Romero L. Preliminary studies on the involvement of biosynthesis of cysteine and glutathione in the resistance to boron toxicity in sunflower plants. Plant Science. 2003;165:811–817. [Google Scholar]
- Sanmartin M, Drogoudi PD, Lyons T, Pateraki I, Barners J, Kanellis AK. Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta. 2003;216:918–928. doi: 10.1007/s00425-002-0944-9. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biology. 2000;3:229–235. [PubMed] [Google Scholar]
- Sotiropoulos TE, Molassiotis A, Almaliotis D, Mouhtaridou G, Dimassi K, Therios I, Diamantidis G. Growth, nutritional status, chlorophyll content, and antioxidant responses of the apple rootstock MM 111 shoots cultured under high boron concentrations in vitro. Journal of Plant Nutrition. 2006;29:575–583. [Google Scholar]
- Wolf B. Improvement in the Azomethine-H method for determination of boron. Communications in Soil Science and Plant Analysis. 1974;5:39–44. [Google Scholar]
- Yamamoto A, Bhuiyan MNH, Waditee R, Tanaka Y, Esaka M, Oba K, et al. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. Journal of Experimental Botany. 2005;56:1785–1796. doi: 10.1093/jxb/eri167. [DOI] [PubMed] [Google Scholar]
- Yokota H, Konishi S. Effect of the formation of sugar borate complex on the growth inhibition of pollen tubes of Camellia sinensis and culture cells of Nicotiana tabacum by toxic levels of borate. Soil Science and Plant Nutrition. 1990;36:275–281. [Google Scholar]
- Yu Q, Osborne L, Rengel Z. Micronutrient deficiency changes activities of superoxide dismutase and ascorbate peroxidase in tobacco plants. Journal of Plant Nutrition. 1998;21:1427–1437. [Google Scholar]