Abstract
Background and Aims
The objectives of this study were to investigate whether a classification of triticale genotypes into drought-tolerant and drought-sensitive types based on field performance trials correlates with a classification based on measurements of some physiological and biochemical parameters in greenhouse conditions. In addition, an examination was carried out of whether ferulic acid, as the main origin of the blue fluorescence produced, contributes to drought tolerance.
Methods
Ten winter triticale genotypes were examined, five known to be drought tolerant and five drought sensitive. Measurements of the osmotic potential, leaf gas exchange, chlorophyll fluorescence, and blue and red fluorescence were performed. In addition, analysis of the total pool of phenolic compounds and ferulic acid as well as the measurements of PAL (l-phenylalanine ammonia-lyase) activity were carried out.
Key Results
In agreement with field trials, three out of five cultivars (‘Lamberto’, ‘Timbo’ and ‘Piano’) were classified as drought tolerant. However, in the case of cultivar ‘Babor’, included in the group of drought-sensitive cultivars, the values obtained for some measured parameters were close to (Fv′/Fm′, phenolics content, osmotic potential) or even better than (non-photochemical quenching, red and blue fluorescence, ferulic acid content) those for drought-tolerant genotypes. Cultivars ‘Imperial’, ‘Ticino’, ‘Trimaran’ and ‘Boreas’ were included in the drought-sensitive group, whereas cultivars ‘Focus’ and ‘Kitaro’ were included in the moderately sensitive group.
Conclusions
The experiments confirmed that the period of flowering, the critical phase for plants as far as water demand is concerned, is suitable for plant screening and differentiation due to their tolerance to drought. The most important criteria which enabled creation of the ranking list of plants, from those sensitive to drought to those tolerant to drought, were the ability to perform the process of osmoregulation, the efficiency of the utilization of excitation energy by the photosynthetic apparatus and the functioning of protective mechanisms involving the level of ferulic acid in leaf tissues.
Key words: Blue fluorescence, chlorophyll fluorescence, drought, ferulic acid, osmotic potential, phenolics, photosynthesis, red and far-red fluorescence, Triticosecale, winter triticale
INTRODUCTION
In many regions water resources for agronomic uses are becoming scarce (Wardlaw, 2002; Llorens et al., 2004; Flexas et al., 2006); therefore, the development of drought-tolerant lines is becoming increasingly more important. Field trials concerning natural drought stress are time-consuming and expensive; furthermore, both space and the seed of genotypes, especially in early generations, are limited, so only a restricted number of genotypes can be tested in any one year. Another problem is that natural drought stress does not occur to the same extent every year, but for the selection process it is advantageous to be able to make selections within a few years under similar field conditions.
Nowadays, efforts are directed to finding new, cheap and physiologically trustworthy tools that can help in both the detection of drought stress and the selection of tolerant genotypes. Studying physiological and biochemical responses to water deficit may help in breeding plant cultivars of high yield and stability under drought conditions (Yordanov et al., 2000).
During the acclimation to soil drought, plants initiate defence mechanisms against water deficit (Medrano et al., 2002; Chaves and Oliveira, 2004). In the present work, fluorescence and spectrofluorescence methods were used to screen plants under drought-stress conditions. Measurement of chlorophyll fluorescence provides information about the function of the photosynthetic apparatus and is useful for stress detection (Bolhàr-Nordenkampf and Öquist, 1993; Schweiger et al., 1996; Yordanov et al., 1997).
It has been shown that water deficit led to an increase in the intensity of the blue fluorescence originating from plant phenolics, mainly from ferulic acid (Lichtenthaler and Schweiger, 1998; Cerovic et al., 2002; Meyer et al., 2003; Morales et al., 2005; Hura et al., 2006). Phenolics can neutralize light falling on the leaves through its transformation into blue fluorescence, which is no longer damaging, and can even be used for photosynthetic quantum conversion (Caldwell et al., 1983; Schweiger et al., 1996; Buschmann and Lichtenthaler, 1998; Bilger et al., 2001). The phenolic compounds are very reactive structures due to the presence of the benzene ring. The benzene ring as a π-electron system can counteract UV and visible radiation (Bilger et al., 1997, 2001). It is known that drought stress predisposes plants to injury of the photosynthetic apparatus through its interaction with UV or/and visible radiation (Garcia-Plazaola and Becerril, 2000). In the genotypes studied here, the contents of ferulic acid, as the main origin of the blue fluorescence, and total phenolics were measured, and the activity of PAL (l-phenylalanine ammonia-lyase), as the key enzyme in the synthesis of phenolics (Strack, 1997), was also analysed.
In the work presented here, attempts were made to estimate whether the division of triticale cultivars into drought-tolerant and drought-sensitive genotypes based on field trials agrees with the division obtained from measurements of some physiological and biochemical parameters in greenhouse conditions, and whether ferulic acid as the main origin of the blue fluorescence may contribute to drought tolerance.
MATERIALS AND METHODS
Plant materials
The experiments were carried out on winter triticale (× Triticosecale, Wittmack) genotypes. Based on field trials, five genotypes were classified as drought sensitive (‘Kitaro’, ‘Focus’, ‘Lamberto’, ‘Timbo’ and ‘Piano’) and five as drought tolerant (‘Babor’, ‘Boreas’, ‘Trimaran’, ‘Ticino’ and ‘Imperial’). Plants were grown during two successive cultivation seasons, 2002–2003. During 2002, field trials were performed at two locations with natural drought stress, in northern Germany and in the middle-east of Germany, whilst in 2003 they were performed at three locations, two in northern and one in southern Germany. Two water regimes were imposed (irrigated vs. non irrigated) with two replications each. Traits such as heading, height, spikes per m2 and grain yield were measured (results not shown). The basis for the selection of the drought-susceptible and drought-tolerant genotypes was the results obtained in 2003, a year of severe drought; however, the results of both the two seasonal experiments were consistent with regard to the grouping of triticale genotypes into tolerant and susceptible. For the experiment in greenhouse conditions, five genotypes with the highest/lowest grain yield in non-irrigated conditions were selected.
Plant growth conditions
Seedlings of each genotype were grown in vermiculite medium to approximately the first leaf stage in a greenhouse before being vernalized for 8 weeks in controlled conditions [maintained at 4 °C with 10 h illumination and with a photosynthetic photon flux density (PPFD) of 200 µmol m−2 s−1]. After vernalization, seedlings of each genotype at the 3-leaf stage were planted in plastic pots (two seedlings per pot) of 15 cm diameter and 18 cm height containing vermiculite as the medium, and were exposed in an air-conditioned greenhouse chamber to a 16 h light/8 h dark photoperiod, a temperature of 23/18 °C (±2 °C) day/night, 60 ± 5 % relative air humidity and a PPFD of 350 µmol m−2 s−1.
For control plants 70 % of FWC (field water capacity) and for water-stressed plants 30–35 % of FWC were applied for 4 weeks. Drought treatment of triticale genotypes took place during heading and flowering. The plants were irrigated with full-strength Hoagland's nutrient solution once per week. The pots were weighed and the amount of the water lost was replaced to maintain the original weight of the pots in each treatment. Each treatment (control and drought) comprised 15 pots. In total, there were 30 samples within each treatment for each genotype. All the pots in each treatment, before measurements, were randomly divided into three subgroups, which were randomly assigned to measurements of physiological and biochemical parameters. The measurements for all genotypes were carried out on flag leaves during flowering.
Osmotic potential
Measurements were carried out with a dew point microvoltmeter (model HR-33T with C-52 sample chambers, Wescor Inc., Logan, UT, USA). Leaf discs (diameter = 5 mm) were taken for analysis from the middle part of leaves and were placed into an Eppendorf tube, frozen in liquid nitrogen and stored at –70 °C. For measurement, leaf samples were thawed at room temperature and the sap from leaf discs was extracted with a syringe and quickly transferred to a leaf chamber. The time needed for the saturation of leaf chambers was set to 40 min. The measurements for each genotype were done in the dew point mode at room temperature on five replicates.
Leaf gas exchange
Leaf gas exchange was measured on the fully expanded leaf using an infrared gas analyser (Li-6400, Portable Photosynthesis System, Lincoln, NE, USA), operated within an open system with a leaf chamber. Net photosynthesis, transpiration rated and stomatal conductance were determined under photosynthetically active radiation (PAR) of 1000 µmol of photons m−2 s−1 and at a CO2 concentration ranging from 380 to 400 ppm. The measurements for each genotype were done on seven replicates.
Chlorophyll fluorescence
Chlorophyll fluorescence was measured on the fully expanded leaf usng a pulse-modulated portable fluorometer FMS2 (Hansatech Instruments, King's Lynn, UK) at ambient temperature in the dark (dark-adapted leaf) and then, after 10 min of irradiation at a PPFD of 500 µmol of photons m−2 s−1 (light-adapted leaf), QP (photochemical quenching coefficient) and NQP (non-photochemical quenching coefficient) were calculated according to Havaux et al. (1991): QP = (Fm′ – Fs)/(Fm′ – Fo′); NQP = (Fm′ – Fm)/(Fm–Fo′). The quantum efficiency of photosystem II (PSII) was calculated according to Genty et al. (1989): ΦPSII = (Fm′ – Fs)/Fm′. The measurements for each genotype were done on five replicates.
Spectrofluorescence
Fluorescence spectra were measured using a spectrofluorometer (Perkin-Elmer LS 50B, Norwalk, CT, USA). Fluorescence emission spectra of the red and far-red fluorescence were recorded between 650 and 800 nm. The leaves were excited at 450 nm. The spectral slit widths were set at 10 nm (excitation and emission). The fluorescence emission spectra of the blue fluorescence were recorded between 380 and 600 nm. The leaves were excited at 350 nm. The slit widths for excitation and emission monochromators were adjusted to 10 nm. The measurements of the blue, red and far-red fluorescence for each genotype were done on five replicates.
l-Phenylalanine ammonia-lyase activity
Measurement of PAL activity was carried according to modified methods described by Peltonen and Karjalainen (1995). All procedures were carried out at + 4 °C. The reaction mixture contained 2·5 mL of 0·2 % solution of l-phenylalanine in 50 mm Tris–HCl (pH 8·5) and 0·5 mL of supernatant. The incubation of the reaction mixture was set at 24 h at 38 °C. Then the absorbance at 290 nm was measured. Enzyme activity was expressed as ng of cinnamic acid produced during 1 min per 1 mg of protein. In enzyme assays, protein contents were determined by the method of Bradford (1976). The measurements for each genotype were done on five replicates.
Phenolic analysis
For both total phenolics and ferulic acid measurements, the lyophilized material was homogenized in 80 % ethanol. The total concentration of phenolics was determined using the Folin–Ciocalteau method of Singleton and Rossi (1965). The absorbance was measured at 760 nm. Chlorogenic acid was used as a standard.
Ferulic acid contents were analysed with a spectrofluorometer (Perkin-Elmer LS 50B, Norwalk, CT, USA). Before measurements, chlorophyll was removed by several extractions with hexane until no green colour was visible. The samples were excited at 243 nm and the detection was carried out at 434 nm. The slit widths for both excitation and emission monochromators were adjusted to 10 nm. The measurements of both total phenolics and ferulic acid concentrations for each genotype were done on five replicates.
Statistical analysis
Statistica 5·0 software for Windows was used. Data presented in the figures are means of five or seven replications ± s.e. Correlations between measured parameters were tested at a probability of P < 0·05, and only statistically significant correlations are presented in the figures.
RESULTS AND DISCUSSION
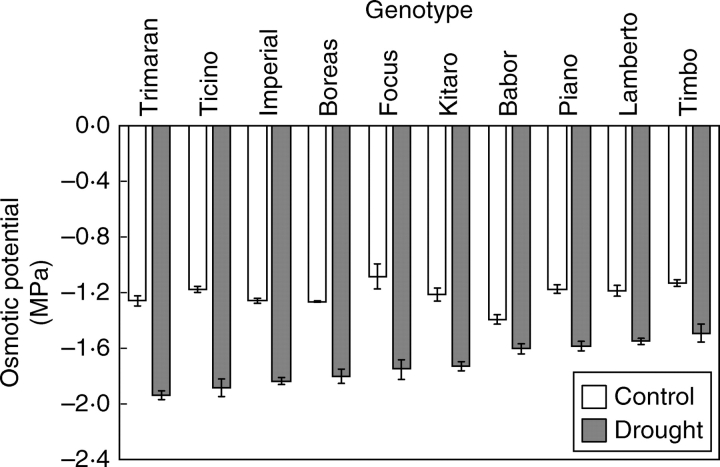
In all tested genotypes a decrease in the osmotic potential (Ψo) during drought stress was observed (Fig. 1). The highest values of Ψo were detected in cultivars ‘Timbo’, ‘Lamberto’, ‘Piano’ and ‘Babor’, and were closer to those of the control plants. For the sensitive cultivar ‘Babor’, Ψo tended to be close to the values for the control, which suggests the existence of efficient osmoregulation.
Fig. 1.
Effect of drought treatment on the leaf osmotic potential (Ψo) of winter triticale genotypes. Data are means ± s.e. of five replicates.
Shangguan et al. (1999) found that the photosynthesis process during drought is possible due to the osmoregulation which affects the state of the leaf stomata and adaptation of the photosynthetic apparatus to drought conditions. Similar results had been obtained earlier in other studies (Matthews and Boyer, 1984; Sen Gupta and Berkowitz, 1988; Verslues and Bray, 2004). Ludlow (1987) and Weng (1993) report a positive correlation between photosynthesis and osmoregulation.
For the tested genotypes, a positive correlation between the photosynthesis rate and Ψo was found (Fig. 2A). In drought conditions, the highest photosynthesis rates were detected for cultivars ‘Piano’, ‘Timbo’ and ‘Lamberto’. A significant correlation was also indicated between the transpiration rate and the osmotic potential (Fig. 2B). The transpiration rate tended to be highest in cultivars ‘Piano’, ‘Timbo’ and ‘Lamberto’. The highest values of stomatal conductance, closest to the control value, were found for the drought-sensitive genotypes ‘Babor’ and ‘Boreas’ (Table 1). This points to severe disturbances in stomatal movement and lack of complete closure in drought conditions. For cultivars tolerant to water deficit, such as ‘Timbo’ and ‘Piano’, the stomatal conductance was high or close to that of the control, respectively. The experiments conducted in long-term drought conditions did not reveal any significant correlation between the stomatal conductance and the photosynthesis rate. This may suggest factors other than stomata-based factors inhibiting the photosynthesis process, most probably linked to the damage of the photosynthetic apparatus (Hura et al., 2006).
Fig. 2.
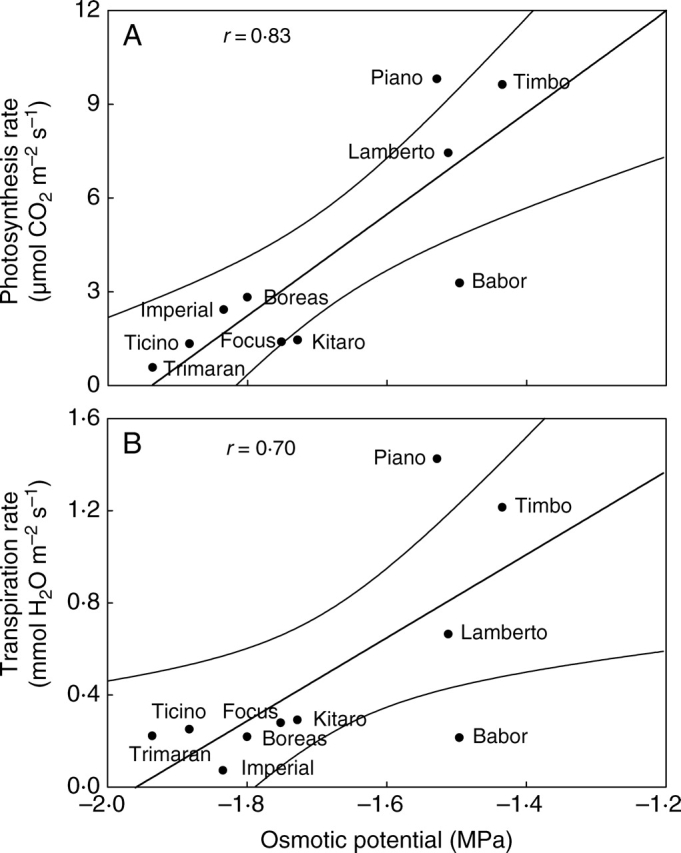
Correlations between photosynthesis rate (A), transpiration rate (B) and osmotic potential (Ψo) for drought-treated genotypes of winter triticale.
Table 1.
Effect of drought stress on the stomatal conductance (mmol CO2 m−2 s−1) of winter triticale genotypes
| Genotypes | Control | Drought |
|---|---|---|
| ‘Kitaro’ | 65·9 ± 1·0 | 54·3 ± 1·8 |
| ‘Imperial’ | 194·2 ± 10·8 | 60·1 ± 3·7 |
| ‘Focus’ | 74·7 ± 5·1 | 61·9 ± 5·2 |
| ‘Ticino’ | 77·4 ± 4·1 | 64·2 ± 6·4 |
| ‘Lamberto’ | 183·0 ± 4·7 | 67·9 ± 6·5 |
| ‘Trimaran’ | 215·3 ± 5·9 | 69·3 ± 6·1 |
| ‘Timbo’ | 190·4 ± 6·1 | 140·7 ± 1·6 |
| ‘Piano’ | 197·3 ± 4·0 | 177·0 ± 5·6 |
| ‘Boreas’ | 297·0 ± 7·1 | 273·3 ± 3·2 |
| ‘Babor’ | 302·3 ± 3·8 | 282·7 ± 1·9 |
Data are means ± s.e. of seven replicates.
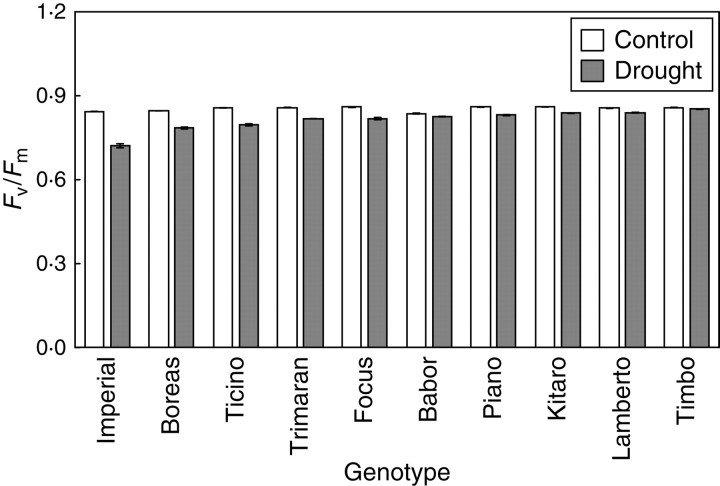
Soil drought did not influence the photochemical efficiency of PSII (Fv/Fm) measured in dark-adapted leaves (Fig. 3). A distinct decrease in this parameter was observed only for the cultivar ‘Imperial’. There are examples of studies showing resistance of PSII to water deficit in leaf tissues (Cornic and Briantais, 1991; Liang et al., 1997; Noguès and Baker, 2000; Cornic and Fresneau, 2002). According to the authors, measurements of Fv/Fm do not provide enough information about PSII in drought conditions because a decrease in the activity of PSII may be a result of photoinhibition.
Fig. 3.
Effect of drought treatment on the maximal efficiency of PSII photochemistry (Fv/Fm) of winter triticale genotypes. Data are means ± s.e. of five replicates.
Statistically significant correlations were found between some parameters related to chlorophyll fluorescence [ΦPSII, QP, ETR (electron transport rate), NQP and Fv′/Fm′] and the Ψo (Fig. 4). Leaf dehydration was accompanied by a decrease in actual fluorescence Fv′/Fm′ for the sensitive genotypes ‘Imperial’, ‘Ticino’, ‘Trimaran’ and ‘Boreas’ (Fig. 4A). A decrease in Fv′/Fm′ may result from low efficiency of the capture of excitation energy by PSII centres and low efficiency of light trapping by the PSII light-harvesting antenna due to photoinhibition. For tolerant cultivars, photoinhibition can be regarded as an adaptation to drought conditions, whereas in the case of sensitive cultivars such as ‘Imperial’, ‘Ticino’, ‘Trimaran’ and ‘Boreas’, it can be regarded as damage to PSII because photoinhibition itself can lead to the degradation of the D1 protein (Barber and Andersson, 1991; Baker and Rosenqvist, 2004).
Fig. 4.
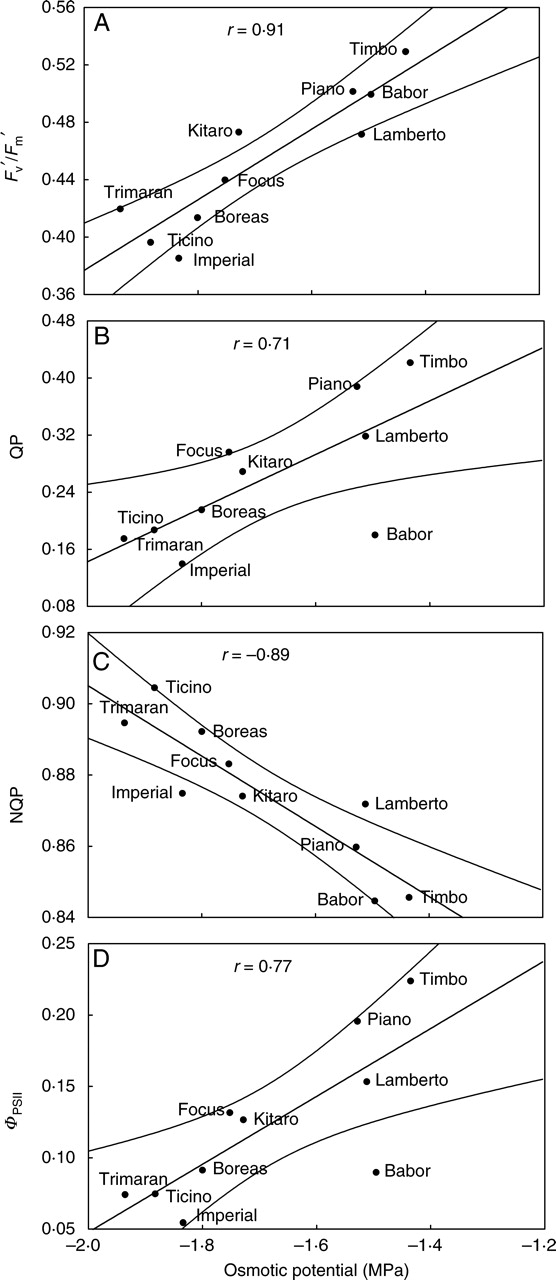
Correlations between (A) actual fluorescence (Fv′/Fm′ ), (B) photochemical quenching coefficient (QP), (C) non-photochemical quenching coefficient (NQP), (D) quantum efficiency of PSII (ΦPSII) and osmotic potential (Ψo) for drought-treated genotypes of winter triticale.
For the genotypes ‘Piano’, ‘Timbo’ and ‘Lamberto’, the highest values of QP under drought were recorded (Fig. 4B). The relatively high values of QP can be explained by the increased accumulation of non-reduced primary electron acceptors (QA) of PSII, which are ready to accept excitation energy in order to pass it further towards other photochemical processes (Maxwell and Johnson, 2000; Noguès and Baker, 2000).
The coefficient of NQP measures energy emitted as heat (Baker and Rosenqvist, 2004), and high emission points to the damaged photosynthetic apparatus (Loggini et al., 1999). The drought-resistant cultivars ‘Timbo’ and ‘Piano’ and drought-sensitive ‘Babor’ showed the lowest values of this parameter (Fig. 4C).
The quantum efficiency of the PSII parameter (ΦPSII) allows determination of the effectiveness of excitation energy utilization by chlorophyll a in the process of photosynthesis (Maxwell and Johnson, 2000; Noguès and Baker, 2000). On the basis of the measurements made, it is possible to distinguish genotypes with the lowest values of ΦPSII, i.e. ‘Imperial’, ‘Ticino’, ‘Trimaran’, and the group with the highest values of this parameter, i.e. ‘Lamberto’, ‘Timbo’ and ‘Piano’ (Fig. 4D). The most important factor influencing the values of ΦPSII could be different contributions of the open reaction centres of PSII to the photochemical processes for different cultivars of winter triticale (Demmig-Adams and Adams, 1992; Loggini et al., 1999).
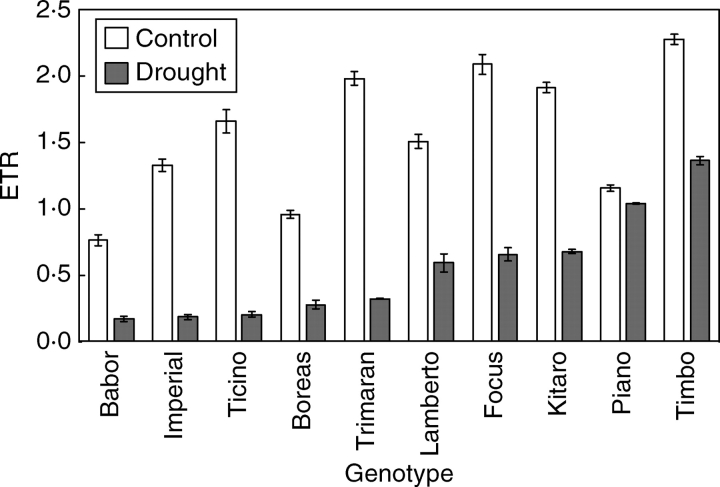
Figure 5 shows changes in the ETR, which reflects the activity of CO2 assimilation. The increase in the value of this parameter, which was noticed for the cultivars ‘Timbo’ and ‘Piano’, reflects the best utilization, among the studied genotypes, of the products of the light phase of photosynthesis in the light-independent phase of photosynthesis (Medrano et al., 2002).
Fig. 5.
Effect of drought treatment on the electron transport rate (ETR) of winter triticale genotypes. Data are means ± s.e. of five replicates.
Statistically significant correlations between the rate of photosynthesis and the chlorophyll fluorescence parameters (Fv′/Fm′, QP, NQP, ΦPSII and ETR) were found (Fig. 6A–E). This result illustrates the fact that in drought conditions the photosynthetic apparatus of the genotypes ‘Piano’, ‘Timbo’ and ‘Lamberto’ were the most efficient in excitation-energy capture by PSII centres followed by the delivery of this energy onwards to the next photochemical reactions in the photosynthesis process, with the lowest loss of the energy as heat. Moreover, for tolerant cultivars, the products of the light phase were utilized more efficiently during the light-independent phase of photosynthesis.
Fig. 6.
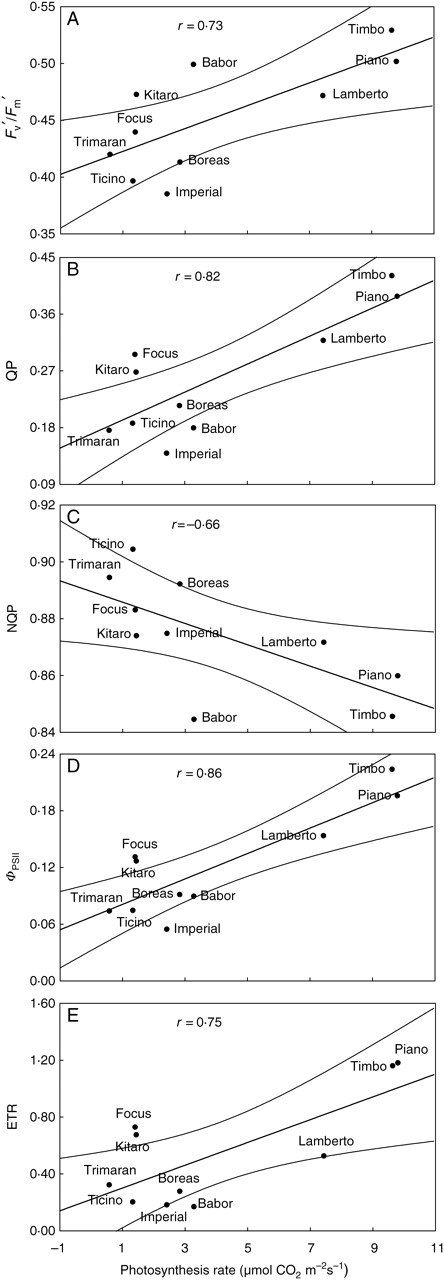
Correlations between (A) actual fluorescence (Fv′/Fm′), (B) photochemical quenching coefficient (QP), (C) non-photochemical quenching coefficient (NQP), (D) quantum efficiency of PSII (ΦPSII), (E) electron transport rate (ETR) and photosynthesis rate for drought-treated genotypes of winter triticale.
Figure 7 shows significant correlations between Ψo and the intensity of the red fluorescence (Fig. 7A) and far-red fluorescence (Fig. 7B). The photosynthetic apparatus is capable of dissipation of the excess energy as heat, but is also able to neutralize high-energy states through the emission of red fluorescence (Schweiger et al., 1996; Hura at al., 2006). This phenomenon may be part of a defence mechanism, but it may also result from damage to the photosynthetic apparatus in the cultivars ‘Ticino’, ‘Trimaran’ and ‘Imperial’.
Fig. 7.
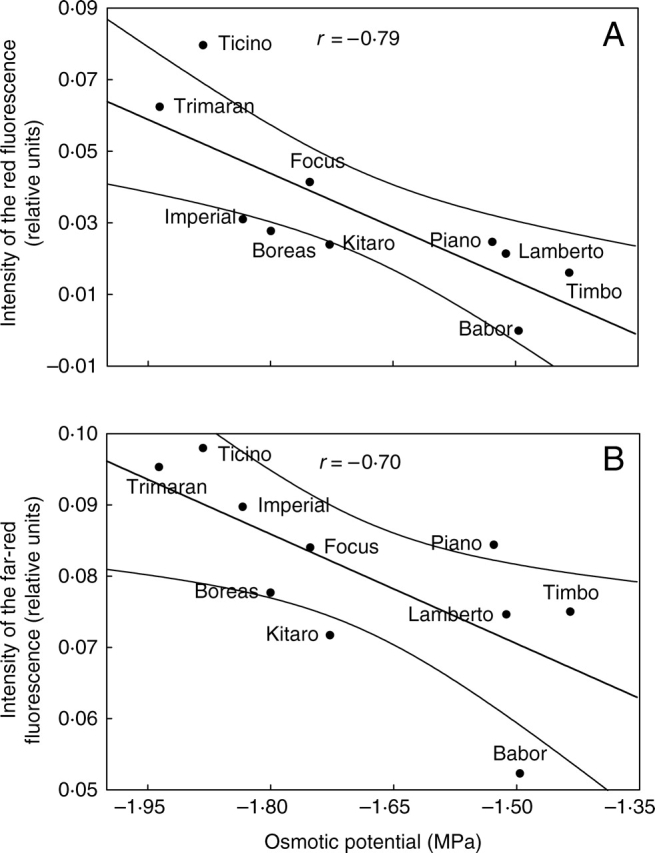
Correlations between (A) emission of red fluorescence, (B) emission of far-red fluorescence and osmotic potential (Ψo) for drought-treated genotypes of winter triticale.
Significant correlations have been found between NQP and the intensity of the red fluorescence (Fig. 8A) and far-red fluorescence (Fig. 8B). The results point to the fact that in drought conditions the cultivars ‘Piano’, ‘Timbo’ and ‘Lamberto’ utilized the excitation energy in the optimal way in comparison with sensitive cultivars. The cultivar ‘Babor’, which was drought sensitive, emitted fluorescence of the lowest intensity in both ranges. The increase in the red fluorescence emission in drought conditions, observed especially for sensitive cultivars, occurs at the cost of the photosynthetic conversion of absorbed light and is related to damage of PSII and light-harvesting complexes and changes in the activity of electron transport molecules (Lichtenthaler, 1996; Šesták and Šiffel, 1997; Buschmann and Lichtenthaler, 1998).
Fig. 8.
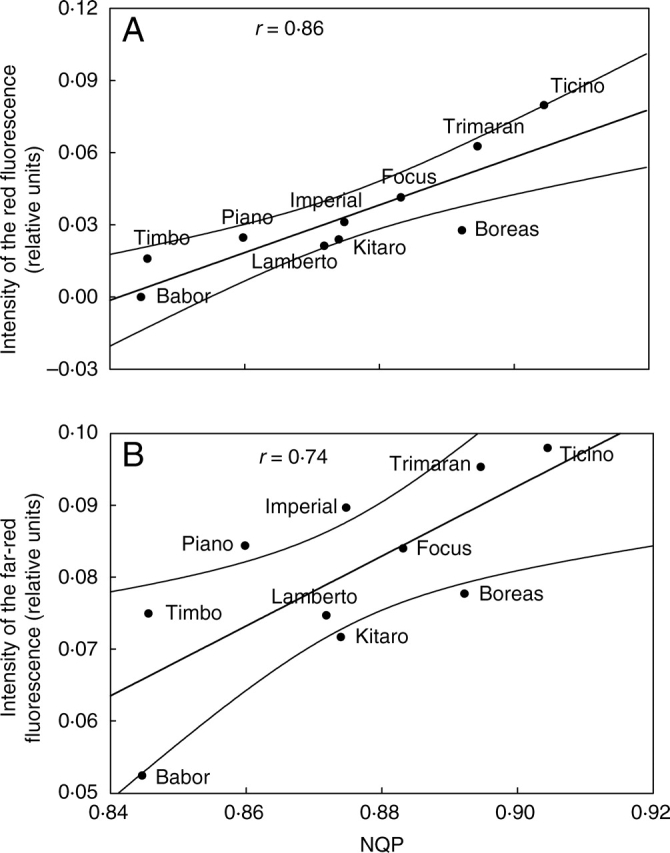
Correlations between (A) emission of red fluorescence, (B) emission of far-red fluorescence and non-photochemical quenching coefficient (NQP) for drought-treated genotypes of winter triticale.
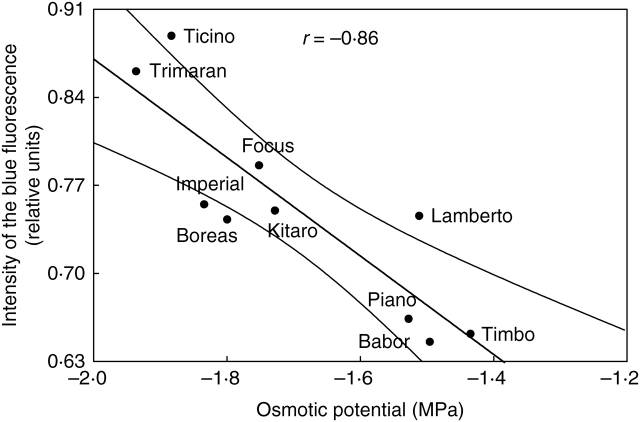
An increase in the emission of blue fluorescence is related to the accumulation of phenolic compounds in the leaf (Schweiger et al., 1996; Bilger et al., 2001). For the sensitive cultivars, the increased emission of blue fluorescence correlated with low Ψo, while the tolerant genotypes showed low emission of blue fluorescence with high Ψo (Fig. 9). Water deficit can induce protective mechanisms against the destructive action of radiation, which could be absorbed and transformed by phenolic compounds into blue fluorescence that, in turn, could be utilized in the photosynthesis process as well as re-emitted as chlorophyll fluorescence (Caldwell et al., 1983; Schweiger et al., 1996; Bilger et al., 1997, 2001; Buschmann and Lichtenthaler, 1998; Lichtenthaler and Schweiger, 1998). Therefore, plants have the possibility to widen the site of the action of radiation on the photosynthetic apparatus in unfavourable conditions where light may be a stress factor. A positive correlation between the ferulic acid content and Ψo was noted (Fig. 10A). A higher ferulic acid content was determined for drought-resistant cultivars than for sensitive cultivars, although the highest content of ferulic acid was detected in the cultivar ‘Babor’, which is sensitive to drought. Ferulic acid, the main source of the blue fluorescence (Lichtenthaler and Schweiger, 1998), can absorb UV and visible radiation (violet and blue light). During the experiments, a statistically significant negative correlation between the intensity of the blue fluorescence and the content of ferulic acid was noted (Fig. 10B), although it was expected that a positive correlation would be found showing that an increase in the ferulic acid content brings about an increase in the emission of blue fluorescence. The negative correlation could be the result of the re-absorption of the emitted fluorescence by phenolic compounds (Lichtenthaler et al., 1998). Moreover, emission of blue fluorescence can be reduced by partial re-absorption of such fluorescence by chlorophylls and carotenoids possessing a blue absorption band (Lang et al., 1996).
Fig. 9.
Correlation between emission of blue fluorescence and osmotic potential of drought-treated genotypes of winter triticale.
Fig. 10.
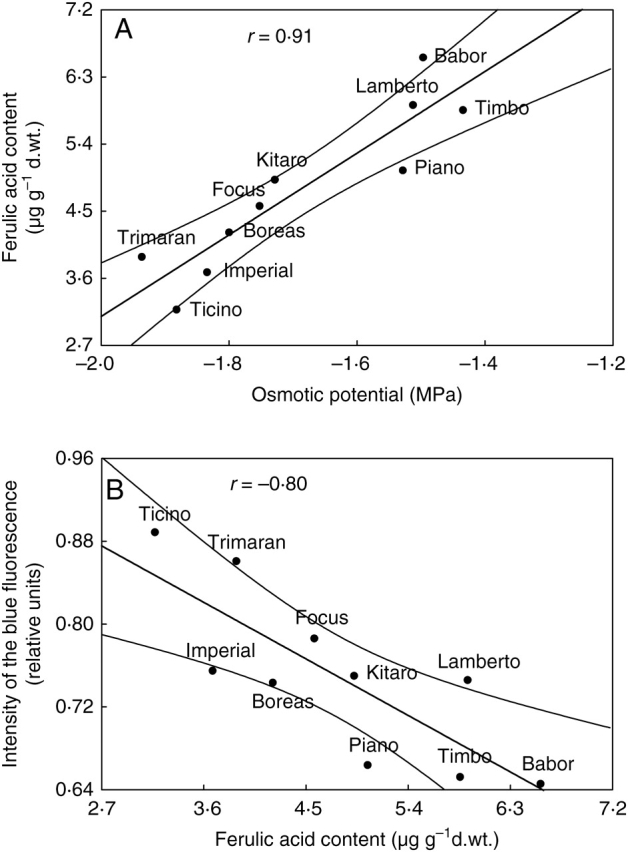
Correlations between (A) ferulic acid content and osmotic potential and (B) emission of blue fluorescence and ferulic acid content for drought-treated genotypes of winter triticale.
Table 2 shows changes in the total pool of phenolic compounds. An increase in the content of phenolics in drought conditions for the sensitive cultivars ‘Trimaran’ and ‘Ticino’ was noticed. The total pool of phenolic compounds did not increase, or increased by only a small amount, in the tolerant cultivars (for ‘Kitaro’ even a small decrease in the total pool of phenolic compounds was observed) at the expense of the synthesis of ferulic acid as an efficient photoprotector.
Table 2.
Effect of drought stress on the content of total phenolics (mg g−1 d. wt) of winter triticale genotypes
| Genotypes | Control | Drought |
|---|---|---|
| ‘Boreas’ | 37·3 ± 1·4 | 27·9 ± 0·5 |
| ‘Imperial’ | 45·9 ± 1·2 | 36·6 ± 1·1 |
| ‘Kitaro’ | 41·7 ± 0·6 | 37·7 ± 1·0 |
| ‘Trimaran’ | 22·3 ± 1·0 | 40·7 ± 0·7 |
| ‘Babor’ | 32·7 ± 0·7 | 41·0 ± 1·2 |
| ‘Lamberto’ | 40·7 ± 0·9 | 41·0 ± 1·1 |
| ‘Piano’ | 39·8 ± 1·2 | 41·9 ± 0·6 |
| ‘Ticino’ | 30·0 ± 0·8 | 45·0 ± 0·5 |
| ‘Timbo’ | 39·5 ± 1·0 | 48·8 ± 1·7 |
| ‘Focus’ | 46·1 ± 1·1 | 54·2 ± 0·8 |
Data are means ± s.e. of five replicates.
An increase in the activity l-PAL, crucial in the synthesis of phenolic compounds, was observed in all cultivars except for ‘Timbo’ and ‘Kitaro’ (Table 3).
Table 3.
Effect of drought stress on PAL activity (ng cinnamic acid min−1mg−1 protein) of winter triticale genotypes
| Genotypes | Control | Drought |
|---|---|---|
| ‘Timbo’ | 2·50 ± 0·13 | 1·69 ± 0·14 |
| ‘Kitaro’ | 2·92 ± 0·17 | 2·60 ± 0·15 |
| ‘Imperial’ | 2·31 ± 0·13 | 2·70 ± 0·27 |
| ‘Babor’ | 1·99 ± 0·19 | 2·75 ± 0·25 |
| ‘Trimaran’ | 1·99 ± 0·17 | 2·84 ± 0·18 |
| ‘Focus’ | 2·58 ± 0·18 | 2·91 ± 0·18 |
| ‘Ticino’ | 2·01 ± 0·11 | 2·96 ± 0·22 |
| ‘Lamberto’ | 2·06 ± 0·06 | 2·99 ± 0·16 |
| ‘Boreas’ | 2·27 ± 0·14 | 3·17 ± 0·19 |
| ‘Piano’ | 2·56 ± 0·15 | 3·85 ± 0·22 |
Data are means ± s.e. of five replicates.
CONCLUSIONS
The results of the physiological and biochemical experiments are in good agreement with the classification into drought-tolerant and drought-sensitive genotypes based on field experiments under natural drought stress. The cultivars ‘Lamberto’, ‘Timbo’ and ‘Piano’ belong to the drought-tolerant group. For the cultivar ‘Babor’, recognized as sensitive, the results of the analyses, especially the measurements of the osmotic potential and chlorophyll fluorescence, were close to and in some cases even better than those of the tolerant cultivars. The lack of agreement with the field trials in this case could be due to the effect of stress conditions other than drought working simultaneously on the plant. In field conditions, it is impossible to eliminate the effects of other negative environmental factors, and the indicator of tolerance examined, for example the crop yield, is a resultant indicator of the influence of many stress factors during the growth and development of plants. The cultivars ‘Imperial’, ‘Ticino’, ‘Trimaran’ and ‘Boreas’ can definitely be classified as drought sensitive on the base of the measurements, while the cultivars ‘Focus’ and ‘Kitaro’ belong to the group of moderately drought-tolerant plants. The experiments were carried out during the flowering phase, which is critical for plants as far as water supply is concerned. This phase seems to be suitable for screening of plants tolerant to drought stress. The most important criteria that enable creation of a ranking list of plants, from sensitive to tolerant to drought, are the ability to carry out osmoregulation (measurements of the osmotic potential) and the effective and optimal utilization of excitation energy by the photosynthetic apparatus in drought conditions. Other important factors in drought tolerance are the protective mechanisms involving, among others, phenolic compounds, especially ferulic acid as one of the most effective photoprotectors whose level can be a reliable indicator of drought tolerance. However, determination of ferulic acid levels and PAL enzyme activities should have been monitored at more than one time point during the stress period to obtain a better understanding of how tolerant and sensitive varieties differ in their response.
Studies carried out in the laboratory based on measurements of the osmotic potential, chlorophyll fluorescence and ferulic acid content enable a greater number of genotypes to be screened in a shorter period of time and may thus become a more economically efficient alternative to long-lasting (several years) and expensive field trials.
LITERATURE CITED
- Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. Journal of Experimental Botany. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- Barber J, Andersson B. Light can be both good and bad for photosynthesis. Trends in Biochemical Sciences. 1991;17:61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Bilger W, Johnsen T, Schreiber U. UV-excited chlorophyll fluorescence as a tool for the assessment of UV-protection by the epidermis of plants. Journal of Experimental Botany. 2001;52:2007–2014. doi: 10.1093/jexbot/52.363.2007. [DOI] [PubMed] [Google Scholar]
- Bilger W, Veit M, Schreiber L, Schreiber U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiologia Plantarum. 1997;101:754–763. [Google Scholar]
- Bolhàr-Nordenkampf HR, Öquist G. Chlorophyll fluorescence as a tool in photosynthesis research. In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf HR, Leegood RC, Long SP, editors. Photosynthesis and production in a changing environment: a field and laboratory manual. London: Chapman & Hall; 1993. pp. 193–206. [Google Scholar]
- Bradford JS. A rapid and sensitive method for the quantitation of microgram quantitaties of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buschmann C, Lichtenthaler HK. Principles and characteristics of multi-colour fluorescence imaging of plants. Journal of Plant Physiology. 1998;152:297–314. [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. Internal filters: prospects for UV-acclimation in higher plants. Physiologia Plantarum. 1983;58:445–450. [Google Scholar]
- Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, Moya I. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant, Cell and Environment. 2002;25:1663–1676. [Google Scholar]
- Chaves MM, Oliveira MM. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany. 2004;55:2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- Cornic G, Briantais JM. Portioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta. 1991;183:178–184. doi: 10.1007/BF00197786. [DOI] [PubMed] [Google Scholar]
- Cornic G, Fresneau Ch. Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany. 2002;89:887–894. doi: 10.1093/aob/mcf064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW , III Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology. 1992;43:599–626. [Google Scholar]
- Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbó M. Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiologia Plantarum. 2006;127:343–352. [Google Scholar]
- Garcia-Plazaola JI, Becerril JM. Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees. 2000;14:485–490. [Google Scholar]
- Genty B, Briantais JM, Adams WW. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Havaux M, Strasser RJ, Greppin H. A theoretical and experimental analysis of the qp and qnp. coefficients of chlorophyll fluorescence quenching and their relation to photochemical and nonphotochemical events. Photosynthesis Research. 1991;27:41–55. doi: 10.1007/BF00029975. [DOI] [PubMed] [Google Scholar]
- Hura T, Grzesiak S, Hura K, Grzesiak MT, Rzepka A. Differences in the physiological state between triticale and maize plants during drought stress and followed rehydration expressed by the leaf gas exchange and spectrofluorimetric methods. Acta Physiologiae Plantarum. 2006;28:433–443. [Google Scholar]
- Lang M, Lichtenthaler HK, Sowinska M, Heisel F, Miehé JA. Fluorescence imaging of water and temperature stress in plant leaves. Journal of Plant Physiology. 1996;148:613–621. [Google Scholar]
- Liang J, Zhang J, Wong M. Can stomatal closure caused by xylem ABA explain the inhibition of leaf photosynthesis under soil drying? Photosynthesis Research. 1997;51:149–159. [Google Scholar]
- Lichtenthaler HK. Vegetation stress: an introduction to the stress concept in plants. Journal of Plant Physiology. 1996;148:4–14. [Google Scholar]
- Lichtenthaler HK, Schweiger J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. Journal of Plant Physiology. 1998;152:272–282. [Google Scholar]
- Lichtenthaler HK, Wenzel O, Buschmann C, Gitelson A. Plant stress detection by reflectance and fluorescence. Annals of the New York Academy of Sciences. 1998;851:271. [Google Scholar]
- Llorens L, Peñuelas J, Estiarte M, Bruna P. Contrasting growth changes in two dominant species of a Mediterranean shrubland submitted to experimental drought and warming. Annals of Botany. 2004;94:843–853. doi: 10.1093/aob/mch211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiology. 1999;119:1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow MM. Contribution of osmotic adjustment to the maintenance of photosynthesis during water stress. Progress in Photosynthesis Research. 1987;4:161–168. [Google Scholar]
- Matthews MA, Boyer JS. Acclimation of photosynthesis to low water potentials. Plant Physiology. 1984;74:161–166. doi: 10.1104/pp.74.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulías J, Flexas J. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany. 2002;89:895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Cartelat A, Moya I, Cerovic ZG. UV-induced blue-green and far-red fluorescence along wheat leaves: a potential signature of leaf ageing. Journal of Experimental Botany. 2003;54:757–769. doi: 10.1093/jxb/erg063. [DOI] [PubMed] [Google Scholar]
- Morales F, Cartelat A, Álvarez-Fernández A, Moya I, Cerovic ZG. Time-resolved spectral studies of blue-green fluorescence of artichoke (Cynara cardunculus L. Var. Scolymus) leaves: identification of chlorogenic acid as one of the major fluorophores and age-mediated changes. Journal of Agricultural and Food Chemistry. 2005;53:9668–9678. doi: 10.1021/jf051842q. [DOI] [PubMed] [Google Scholar]
- Nogués S, Baker NR. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. Journal of Experimental Botany. 2000;51:1309–1317. doi: 10.1093/jxb/51.348.1309. [DOI] [PubMed] [Google Scholar]
- Peltonen S, Karjalainen R. Phenylalanine ammonia-lyase activity in barley after infection with Bipolaris sorokiniana or treatment with its purified xylanase. Journal of Phytopathology. 1995;143:239–245. [Google Scholar]
- Schweiger J, Lang M, Lichtenthaler HK. Differences in fluorescence excitation spectra of leaves between stressed and non-stressed plants. Journal of Plant Physiology. 1996;148:536–547. [Google Scholar]
- Sen Gupta A, Berkowitz GA. Chloroplast osmotic adjustment and water stress effects on photosynthesis. Plant Physiology. 1988;88:200–206. doi: 10.1104/pp.88.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šesták Z, Šiffel P. Leaf-age related differences in chlorophyll fluorescence. Photosynthetica. 1997;33:347–369. [Google Scholar]
- Shangguan Z, Shao M, Dyckmans J. Interaction of osmotic adjustment and photosynthesis in winter wheat under soil drought. Journal of Plant Physiology. 1999;154:753–758. [Google Scholar]
- Singleton VS, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagent. American Journal of Enology and Viticulture. 1965;16:144–157. [Google Scholar]
- Strack D. Phenolic metabolism. In: Dey PM, Harborne JB, editors. Plant biochemistry. London: Academic Press; 1997. pp. 387–416. [Google Scholar]
- Verslues PE, Bray EA. LWR1 and LWR2 are required for osmoregulation and osmotic adjustment in Arabidopsis. Plant Physiology. 2004;136:2831–2842. doi: 10.1104/pp.104.045856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw IF. Interaction between drought and chronic high temperature during kernel filling in wheat in a controlled environment. Annals of Botany. 2002;90:469–476. doi: 10.1093/aob/mcf219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JH. Photosynthesis of different ecotypes of Miscantus spp. as affected by water stress. Photosynthetica. 1993;29:43–48. [Google Scholar]
- Yordanov I, Tsonev T, Goltsev V, Kruleva L, Velikova V. Interactive effect of water deficit and high temperature on photosynthesis of sunflower and maize plants. 1. Changes in parameters of chlorophyll fluorescence induction kinetics and fluorescence quenching. Photosynthetica. 1997;33:391–402. [Google Scholar]
- Yordanov I, Velikova V, Tsonev T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica. 2000;38:171–186. [Google Scholar]