Table 1.
One-Pot Synthesis of (Z)-Trisubstituted Allylic Alcohols
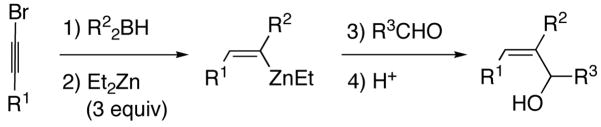 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3CHO | yield (%) | product |
| 1 | n-Bu | Et | CH2O | 70a | 1 |
| 2 | CH2OBn | Et | CH2O | 61a | 2 |
| 3 | n-Bu | Et | Me2CHCH2CHO | 61 | 3 |
| 4 | (CH2)2OTBDPS | Et | Me2CHCH2CHO | 71 | 4 |
| 5 | (CH2)2OTBDPS | Et | p-Cl-C6H4-CHO | 84 | 5 |
| 6 | CH2OBn | Et | p-Cl-C6H4-CHO | 65 | 6 |
| 7 | (CH2)2OTBS | Et | p-Me-C6H4-CHO | 82 | 7 |
| 8 | (CH2)2OTBS | Et | p-Cl-C6H4-CHO | 84 | 8 |
| 9 | n-Bu | Cy | p-Me-C6H4-CHO | 60 | 9 |
| 10 | n-Bu | Cy | o-MeO-C6H4-CHO | 63 | 10 |
Two equiv para formaldehyde added without removal of the volatile materials.
