Table 5.
Diastereoselective Synthesis of α-Methyl Substituted Allylic Alcohols
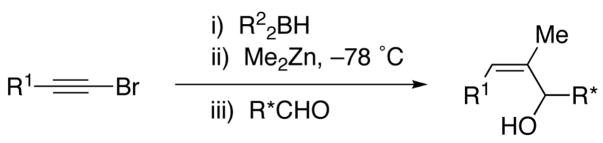 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R1 | R2 | RCHO | yield (%) | dra | product | major product |
| 1 | n-Bu | Cl | 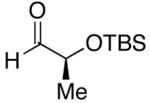 |
68 | >20:1 | 41 | 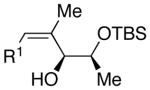 |
| 2 | (CH2)3Cl | Cl | 67 | >20:1 | 42 | ||
| 3 | (CH2)2OTBDPS | Cl | 78 | >20:1 | 43 | ||
| 4 | n-Bu | Me | 78 | >20:1 | 41 | ||
| 5 | (CH2)2OTBDPS | Me | 75 | >20:1 | 43 | ||
| 6 | n-Bu | Cl | 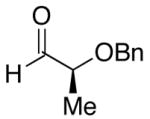 |
51 | >20:1 | 44 | 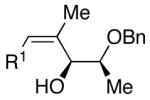 |
| 7 | (CH2)2OTBDPS | Cl | 57 | 17:1 | 45 | ||
| 8 | n-Bu | Br | 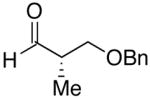 |
70 | 17:1 | 46 | 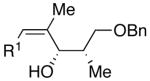 |
| 9 | CH2OTBDPS | Br | 63 | 9–13:1 | 47 | ||
| 10 | (CH2)2OTBDPS | Br | 59 | 11:1 | 48 | ||
| 11 | (CH2)2OTBDPS | Cl | 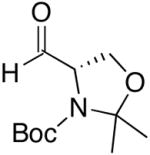 |
47 | >20:1 | 49 | 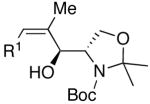 |
| 12 | (CH2)2OTBDPS | Cl | 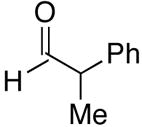 |
55 | 1:4 | 50 | 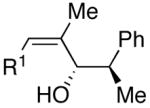 |
Diastereomeric ratio (anti-Felkin : Felkin) based on 1H NMR of crude product. Relative stereochemistry was determined by X-ray crystallography, Mosher ester analysis, or Heathcock’s analysis. See Supporting Information for details.
