Figure 2.
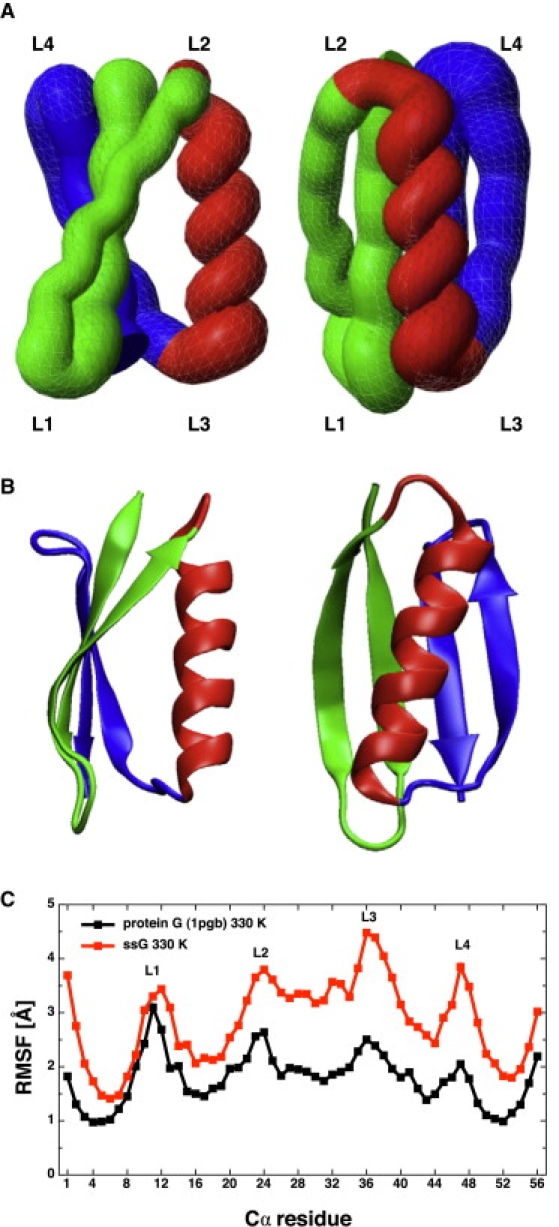
Comparison of the molten-globule state extracted from the simulations of protein ssG (A) and the x-ray structure of protein G (B). The N-terminal β-hairpin, central α-helix, and C-terminal β-hairpin are in green, red, and blue, respectively. The tubelike rendering in panel A was generated using MOLMOL (48) and 100 snapshots from the most populated mesostate. Note that the topology of protein ssG is the same as the one of the wild-type protein but the lack of long side chains and specific contacts in the former results in a flatter β-sheet and a slightly different orientation of the α-helix with respect to the β-sheet. (C) Comparison of Cα root mean-square fluctuations (RMSF). For both proteins, the RMSF values are calculated at the same temperature (330 K) and by averaging over the same number of 1-ns intervals extracted from trajectory segments during which the proteins are in the folded state (i.e., RMSD <5.0 Å from the x-ray structure and the center of the most populated mesostate for the wild-type and protein ssG, respectively).
