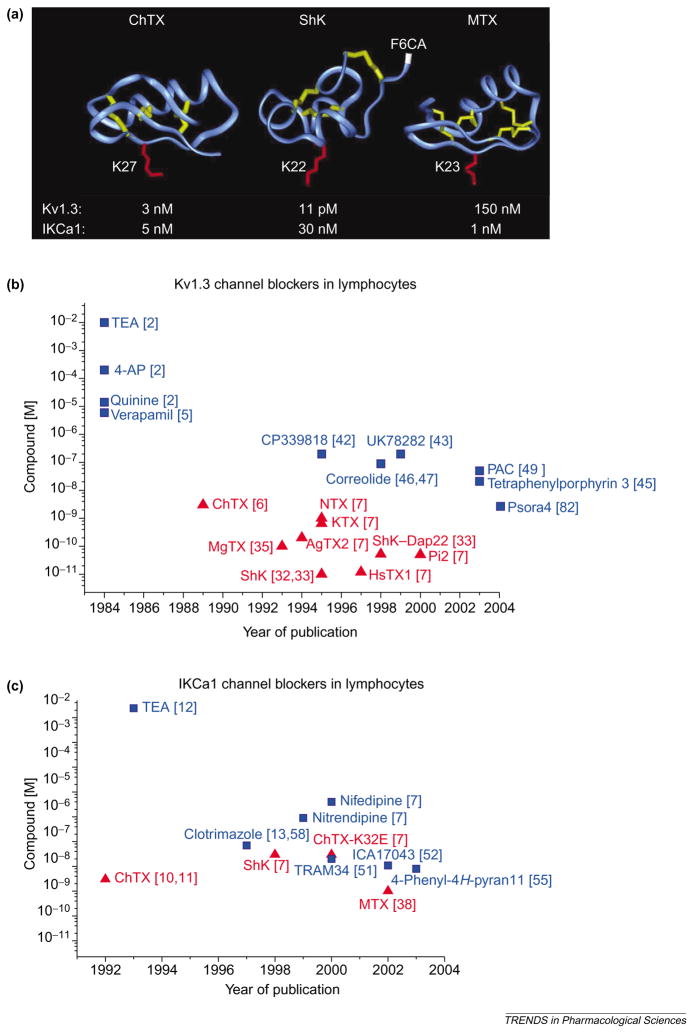
Figure 3.
(a) Backbone structures of charybdotoxin (ChTX), Stichodactyla helianthus toxin (ShK) and maurotoxin (MTX), which are peptide inhibitors of voltage-dependent Kv1.3 channels and intermediate-conductance Ca2+ -activated IKCa1 channels. The crucial lysine that occludes the channel pore is highlighted in red in each structure: Lys27 in ChTX; Lys22 in ShK; and Lys23 in MTX. The disulfide bonds are highlighted in yellow. Arg1 in ShK, to which the fluorophore fluorescein-6-carboxylic acid (F6CA) is attached, is highlighted in white. Such fluorophores attached to specific inhibitors can be used to visualize these K+ channels. (b,c) Plots of potency versus year of publication for peptide (red; triangles) and small-molecule (blue; squares) blockers of Kv1.3 (b) and IKCa1 (c) channels in lymphocytes are shown. Abbreviations: AgTX2, agio-toxin-2; 4-AP, 4-aminopyridine; ChTX-K32E, charybdotoxin derivative with glutamate at position 32 in place of the native lysine; HsTX1, Heterometrus spinnifer toxin 1; KTX, kaliotoxin; MgTX, margatoxin; NTX, noxiustoxin; PAC, 4-phenyl-4-[3-(2-methoxyphenyl)-3-oxo-2-azaprop-1-yl]cyclohexanone; Pi2, Pandinus imperator toxin 2; ShK–Dap22, Stichodactyla helianthus toxin with diaminopropionic acid introduced at position 22 in place of the native lysine; TEA, tetraethylammonium chloride. See Chemical names. (Data are from [2,5–7,10–13,32,33,35,38,42,43,45–47,49,51,52,55,58,82].)