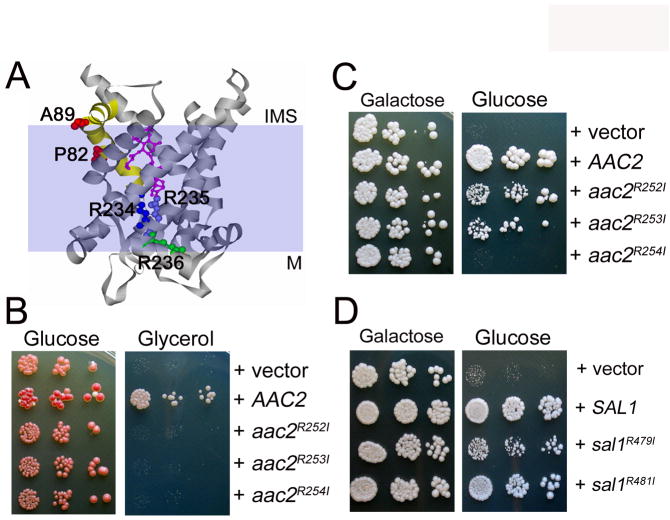
Fig. 9.
Mutagenic bisection of Aac2p and Sal1p. (A) Projected localization of Pro82 and Ala89 (red) on the transmembrane helix 2 (yellow), and the nucleotide-binding Arg234 (dark blue), Arg235 (light blue) and Arg236 (green) in the crystal structure of bovine Ant1 bound by carboxyatractyloside (magenta) (Pebay-Peyroula et al. 2003). These amino acids correspond to Pro89 and Ala96 of Aac1p and to Arg252, 253 and 254 of Aac2p respectively. M, matrix; IMS, inter-membrane space. (B) Complementation of the aac2 (but SAL1) mutant for respiratory growth by mutant aac2 alleles. CS341 was transformed with the centromeric pCXJ24 (LEU2) expressing AAC2, aac2R252I, aac2R253I and aac2R254I respectively and the transformants were tested for respiratory growth on YPGly. (C) aac2R252I and aac2R253I, but not aac2R254I, retain the V function. Transformants of CS494-2B (aac2Δ, GAL10-AAC2, sal1-1) were tested on YPD to deplete the expression of AAC2. (D) Arg479 and Argh481 are not essential for Sal1 function. Transformants of CS494-2B expressing the sal1R479I and sal1R481I alleles were tested for cell viability on YPD.