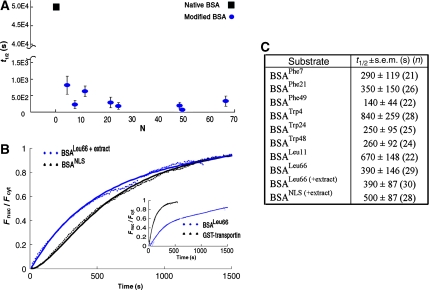
Figure 4.
Nuclear import kinetics of BSA modified by either hydrophobic amino acids or by NLSs and of an NTR. (A) A plot of t1/2 values measured for all BSA derivatives used in this study (blue circles) versus N—the estimated number of hydrophobic moieties attached to the protein. With our experimental setup and timeframe of the measurements, we could not accurately determine the nuclear entry rate of unmodified BSA because it was too low. It has been estimated that BSA traffics to the nucleus at least 600 times slower than transportin (Ribbeck and Gorlich, 2001). Combining this with our data, we estimate that native BSA enters the nucleus with a t1/2 of about 50 000 s, under the experimental conditions we used. This latter value is marked in the graph by a black square (note the break in the y axis). (B) Comparison between nuclear import kinetics of BSALeu66 and BSA derivatized by NLSs, which serve as substrates for the transport receptor complex importin α/β. In these experiments, the cells were supplemented by cytoplasmic extract and an energy regenerating system. (Inset) Nuclear import kinetics of BSALeu66 as compared with that of the (unloaded) nuclear import receptor transportin (fused to GST), both measured in unsupplemented cells. Data describing the nuclear import of the transport receptor were taken from Naim et al (2007). (C) t1/2 values derived for nuclear transport of BSA derivatives used in this study. The numbers in parenthesis denote the number of cells used in the analysis.