Abstract
OBJECTIVE
Fulminant type 1 diabetes is characterized by the rapid onset of severe hyperglycemia and ketoacidosis, with subsequent poor prognosis of diabetes complications. Causative mechanisms for accelerated β-cell failure are unclear.
RESEARCH DESIGN AND METHODS
Subjects comprised three autopsied patients who died from diabetic ketoacidosis within 2–5 days after onset of fulminant type 1 diabetes. We examined islet cell status, including the presence of enterovirus and chemokine/cytokine/major histocompatibility complex (MHC) expressions in the pancreata using immunohistochemical analyses and RT-PCR.
RESULTS
Immunohistochemical analysis revealed the presence of enterovirus-capsid protein in all three affected pancreata. Extensive infiltration of CXCR3 receptor–bearing T-cells and macrophages into islets was observed. Dendritic cells were stained in and around the islets. Specifically, interferon-γ and CXC chemokine ligand 10 (CXCL10) were strongly coexpressed in all subtypes of islet cells, including β-cells and α-cells. No CXCL10 was expressed in exocrine pancreas. Serum levels of CXCL10 were increased. Expression of MHC class II and hyperexpression of MHC class I was observed in some islet cells.
CONCLUSIONS
These results strongly suggest the presence of a circuit for the destruction of β-cells in fulminant type 1 diabetes. Enterovirus infection of the pancreas initiates coexpression of interferon-γ and CXCL10 in β-cells. CXCL10 secreted from β-cells activates and attracts autoreactive T-cells and macrophages to the islets via CXCR3. These infiltrating autoreactive T-cells and macrophages release inflammatory cytokines including interferon-γ in the islets, not only damaging β-cells but also accelerating CXCL10 generation in residual β-cells and thus further activating cell-mediated autoimmunity until all β-cells have been destroyed.
Fulminant type 1 diabetes is characterized by abrupt onset of severe hyperglycemia and ketoacidosis preceded by flu-like symptoms including fever, abdominal pain, and headache (1–3). Due to the rushed clinical course in most cases, patients with fulminant type 1 diabetes are sometimes untreated until becoming comatose and/or entering a critical, life-threatening state (4). Endogenous insulin secretion is completely abolished over time and diabetic microangiopathies develop over a short duration (5,6). The mechanisms underlying the aggressive and rapid destruction of β-cells have remained one of the major questions regarding this subtype of type 1 diabetes. However, in situ human data on affected islets and pancreas and possible mechanisms have been completely lacking for fulminant type 1 diabetes.
Viral infection with subsequent immunological mechanisms represents one of the leading candidates for destruction of β-cells in fulminant type 1 diabetes (3,7). Some studies on the mouse model of lymphocytic choriomeningitis virus–induced type 1 diabetes have demonstrated that islet β-cells can be destroyed as follows: within 1 day after virus infection, CXC chemokine ligand 10 (CXCL10) (8), a key chemoattractant for activated T-cells and macrophages, is produced in β-cells and secreted from islets (9). Activated T-cells bearing the receptor for CXCL10, named CXCR3 (8), infiltrate and accumulate in islets secreting CXCL10 (10). Accumulated T-cells at the islets then destroy β-cells through cell-mediated mechanisms (11). With this mechanism, CXCL10 is necessary and sufficient for accelerated T-cell response with complete β-cell destruction and resulting type 1 diabetes (10,12,13). We have recently found that serum CXCL10 levels are increased at the onset of fulminant type 1 diabetes, suggesting a crucial role of the CXCL10-CXCR3 axis in the aggressive β-cell destruction in this syndrome (14). We therefore examined in situ status with regard to enterovirus infection, CXCL10-CXCR3 axis, major histocompatibility complex (MHC) molecule expression, and islet dysfunction in pancreata from patients with fulminant type 1 diabetes who died due to diabetic ketoacidosis within 2–5 days after outset of flu-like symptoms. Our in situ findings for affected pancreata provide new insights into understanding the pathogenesis of and developing interventional strategies against human type 1 diabetes.
RESEARCH DESIGN AND METHODS
Patients
Case 1.
A 14-year-old boy with type 1 diabetes in a ketoacidotic coma was brought to our hospital and died 20 min later. He had developed headache and high fever (∼38°C) 5 days earlier, with sudden onset of polyuria and polydipsia 1 day before arrival. Blood glucose and hemoglobin A1C levels were 70.3 mmol/l and 7.9%, respectively. Blood pH was 6.98 and plasma level of 3-hydroxybutyrate was 64,000 μmol/l. Serum C-peptide levels were <0.017 nmol/l. Negative results were obtained for autoantibody against GAD (GADAb) and IA-2Ab. Serum elastase-1 and amylase levels were 4.4 and 8.9 times above the upper limit of normal, respectively. HLA-DRB1 and DQB1 genotypes in this patient were *0405/*0803 and *0401/*0601, respectively.
Case 2.
A 25-year-old man with diabetic ketoacidosis arrived at the hospital and died 40 min later. He had experienced symptoms of nausea and epigastralgia for 2 days before becoming comatose. Blood glucose concentration was 85.5 mmol/l, and A1C level was 5.1%. Blood gas analysis revealed acidosis (pH 6.91). Serum elastase-1 concentration was 3.4 times the upper limit of normal. Negative results were obtained for GADAb, IA-2Ab, and autoantibodies against insulin. The patient's HLA-DRB1 and DQB1 genotypes were *0101/*0405 and *0501/*0401, respectively. This case was partly reported previously (3).
Case 3.
A 29-year-old man who collapsed with diabetic ketoacidosis was admitted to our hospital and died 1 h after arrival. Two days earlier he had experienced slight fever, nausea, and vomiting, followed the next day by severe thirst and polyuria. On the day of admission, his family had found him in a comatose state. Blood glucose level was 44.4 mmol/l, A1C level was 5.9%, blood pH was 6.99, and pancreatic-isoamylase level was 40 times the upper limit of normal. HLA-DRB1 and DQB1 genotypes in this patient were *0405/*0901 and *0401/*0303, respectively.
Pancreatic tissues from 7 male patients with pancreatitis ([means ± SD] aged 61 ± 20 years) and 10 nondiabetic male patients (aged 62 ± 10 years) with gastric carcinoma and had undergone partial pancreatectomy were used as inflammation control subjects and nondiabetic control subjects, respectively. In addition, pancreatic tissue from an autopsied patient (a 56-year-old woman who died due to cerebral infarction) with slowly progressive insulin-dependent type 1 diabetes (15) was also examined for presence of enterovirus and CXCL10 expression in the pancreas. She had been treated with insulin and had shown diminished urinary C-peptide secretion (1.1 nmol/day) and high serum GADAb titer (12.5 units/ml [221.4 WHO units/ml]).
Detection of viral RNA in pancreatic tissues.
RNAs were extracted from two 5-μm paraffin sections using a RecoverAll total nucleic acid isolation kit (Ambion, Austin, TX) according to the protocol defined by the manufacturer. Nested RT-PCR targeting the 5′ nontranslated region and VP1 region was performed using the primers described previously (16–19). RT-PCR for CXCL10 and interferon-γ was performed using the primer described previously (20,21).
Immunostaining, immunofluorescent staining and morphometric analyses.
Methods for immunohistochemical and morphometric analyses have been reported previously (22). In brief, serial sections (5 μm) were cut from 5% formaldehyde-fixed paraffin-embedded specimens, stained with hematoxylin and eosin, and then stained using indirect immunoperoxidase techniques and double- or triple-immunofluorescence techniques. Serial sections (5 μm) were deparaffinized, rehydrated, and subjected to antigen unmasking with citrate buffer (pH 6.0). Sections were processed using an Envision+ kit (Dako, Carpinteria, CA) or ABC Staining System (Santa Cruz Biotechnology, Santa Cruz, CA), then visualized with diaminobenzidine tetrahydrochloride or 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium chloride according to the instructions from the manufacturer. Primary antibodies used in this study were guinea pig anti-swine insulin (Dako), rabbit anti-human glucagon (Dako), mouse monoclonal anti-enterovirus VP1 peptide (clone 5-D8/1; Novocastra, Newcastle Upon Tyne, U.K.; this antibody recognizes an epitope mapped to residue 40–48 at the NH2-terminus of VP1 of enterovirus protein [23] and reacts with 36 enteroviral serotypes [24]), mouse monoclonal anti-CD8 (clone 144B; Dako), mouse monoclonal anti-CD4 (IF4; Novocastra), mouse monoclonal anti-CD56 (clone CD564; Novocastra), rabbit monoclonal anti-CD11c (EP1347Y; Abcam, Cambridge, U.K.), goat polyclonal anti-CXCL10 (R&D Systems, Minneapolis, MN), rabbit polyclonal anti-interferon-γ (Santa Cruz Biotechnology), mouse monoclonal anti–interferon-α (NYRhIFN-a; Abcam), rabbit anti–2′,5′-oligoadenylate synthetase-like protein (HPA001474; Sigma), mouse monoclonal anti–interferon-γ (clone 25718; R&D Systems), mouse monoclonal anti-CXCR3/CD183 (clone 1C6; BD Bioscience, San Jose, CA), mouse monoclonal anti-CD68 (clone PG-M1; Dako), mouse monoclonal anti–HLA class-I (clone EMR8-5; Hokudo, Sapporo, Japan), and mouse monoclonal anti–HLA-DR (clone TAL.1B5; Dako).
For immunofluorescent staining, sections were processed as described above then incubated with 7-amino-4-methylcoumarin-3-acetic acid-, Texas Red-, fluorescein isothiocyanate– or Rhodamin Red–conjugated secondary antibodies (all from Jackson ImmunoResearch Laboratories, West Grove, PA). Stained sections were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and analyzed on an IX71 microscope (Olympus, Tokyo, Japan). Phenotyping of mononuclear cells that had infiltrated islets was performed using serial pancreas sections, and more than 23 islets from each patient and control were examined. Immunostainings were carried out at least three times for each section of the pancreas. Some pancreatic sections were processed with isotype-matched control immunoglobulins (mouse IgG1, κ [DAK-GO1; Dako]; mouse IgG2a, κ [DAK-GO5; Dako]; and rabbit IgG [X0903; Dako]) or in the absence of primary antibody to confirm the specificity of immunostaining. We confirmed that each primary antibody was specific for each antigen (supplementary Fig. S1 [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0091/DC1). Sections were processed for immunostaining in the same run.
Morphometric analyses were performed using NIH Image software (http://rsb.info.nih.gov/nih-image/). Photographs of histological specimens for each case were taken at magnifications of ×200 and ×400 for analysis. Twenty-two images were examined for each patient compared with 34 for each control subject. Percentage islet area was obtained by dividing the islet area by the area of the entire section examined.
The percentage β-cell area and percentage α-cell area were calculated by dividing each cell area by the area of the corresponding islet. β-Cell volume and α-cell volume were calculated using percentage islet area multiplied by percentage β-cell area or percentage α-cell area, respectively. According to previous criteria (25), insulitis was defined as infiltration of two or more mononuclear cells into the islet.
Serum CXCL10 assay.
We examined serum CXCL10 levels in two fulminant type 1 diabetic patients (cases 1 and 2) using enzyme-linked immunosorbent assay, as previously described (26). Serum samples were obtained on arrival and stored at −80°C until assay.
Ethics.
All procedures used in this study were approved by the ethical committee of the University of Yamanashi.
Statistical analysis.
Differences in variables between groups were compared using the Student's t test. Fisher's exact test was used to compare the frequencies of positive immunostainings. Values are expressed as means ± SD, unless otherwise mentioned.
RESULTS
Enterovirus in the pancreas.
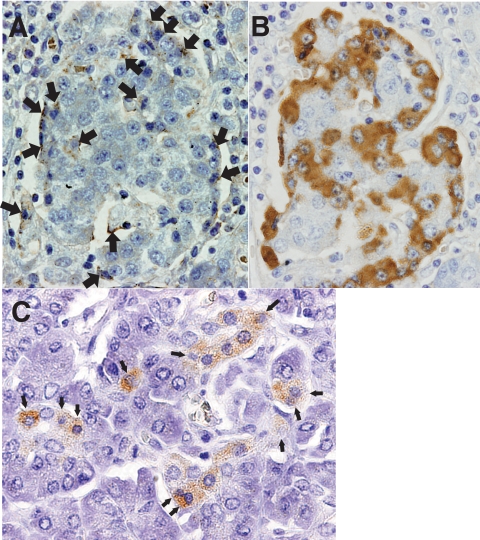
Immunohistochemical staining showed the presence of enterovirus capsid protein (VP1) in the pancreas from all three patients with fulminant type 1 diabetes (Fig. 1A–C). Some proportion of islet cells was positive for VP1 (Fig. 1A and B). Some VP1-positive acinar cells showed degenerating pathological features (Fig. 1C). The number of VP1-positive cell clusters on examined sections was 892/cm2, 470/cm2, and 752/cm2 in cases 1, 2, and 3, respectively. No VP1-positive cell clusters were found in the 10 nondiabetic control subjects (P = 0.004), 7 patients with pancreatitis (P = 0.008) (supplementary Fig. S2), or in the patient with slowly progressive type 1 diabetes.
FIG. 1.
A: Immunohistochemical demonstration of enterovirus-associated VP1 antigen in pancreatic islets (brown, arrows). Cells with shrunken and dark nuclei (arrows) suggestive of pyknosis, a sign of cell death, were observed (×400, case 1). B: Immunohistochemical staining for glucagon in serial sections of (A) (×400). Comparing (A) and (B) indicates enterovirus VP1 antigen residing on islet cells. C: Homogeneous staining for VP1 was observed in pancreatic acinar cell clusters (brown) with shrunken and darkly staining nuclei suggestive of pyknosis (arrows) (×400). (A high-quality digital representation of this figure is available in the online issue.)
We were unable to detect the enterovirus sequence, and we could not amplify 18S rRNA and/or glyceraldehyde phosphate dehydrogenase (GAPDH) cDNAs from the pancreatic sections of diabetic patients, although we could detect 18S rRNA and/or GAPDH sequences from control pancreata. We therefore assumed that enterovirus RNAs had already degraded.
CD8+ T-cells, macrophages, and CD11c+ dendritic cells in the pancreas.
Marked mononuclear cell (MNC) infiltration into islets (insulitis) and around islets (peri-insulitis) was observed in all three cases with fulminant type 1 diabetes (Fig. 2A–C). Frequency of insulitis per examined islet was almost 100% in all three cases (Table 1). Islet volume and β-cell volume were markedly decreased (Table 1). α-Cell volume was decreased in case 1. Some exocrine pancreatic tissues, including acinar and ductal cells, were also surrounded by MNCs.
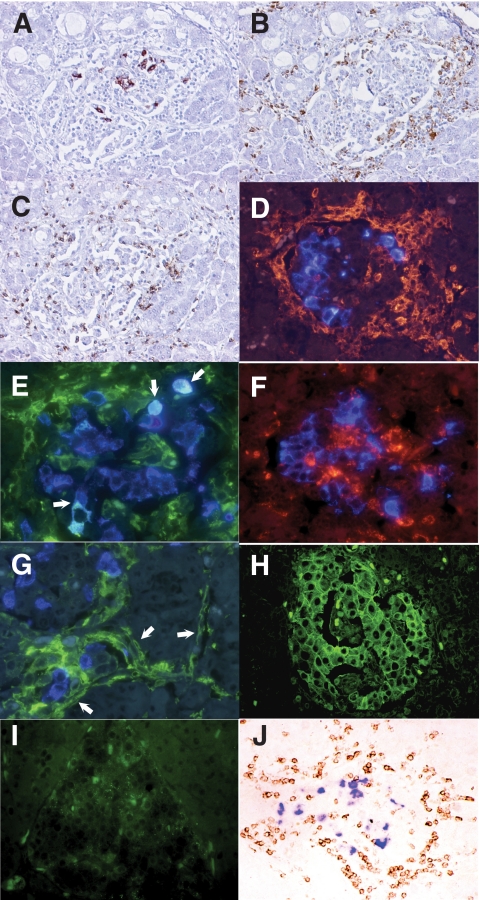
FIG. 2.
Mononuclear cell infiltration into islets with residual β-cells (A) (brown), macrophages (B) (brown), and CD8+ T-cells (C) (brown) (×200, serial sections of case 1). D: Double immunofluorescent staining for CD11c+ dendritic cells (red) and insulin (blue) demonstrates that some dendritic cells surrounded and infiltrated into islets (×400, case 1). E: Double immunofluorescent staining for insulin (blue) and MHC class II antigen (green) demonstrates that some residual β-cells aberrantly express MHC class II molecules (light blue, arrows) (×400, case 1). F: Double immunostaining for CD68+ macrophages (red) and insulin (blue). Insulin was not stained in macrophages (×400, case 1). G: Double immunofluorescent staining for MHC class II molecules (green) and α-cells (blue) demonstrates aberrant expression of MHC class II molecules on vascular endothelium around and within the islets (arrows) (×400, case 1). H: Immunofluorescent staining demonstrates hyperexpression of MHC class I molecules (green) on islet cells (×200, case 1). I: Faint staining of MHC class I molecules (green) were observed on some nondiabetic control islet-cells (×200). J: Double immunostaining of the pancreatic section stained for CXCL10 (purple) and CXCR3 (brown). CXCR3-positive cells have infiltrated islet cells expressing CXCL10 (×200, case 1). (A high-quality digital representation of this figure is available in the online issue.)
TABLE 1.
Results on morohometric analysis, frequency of insulitis, and phenotypic analysis on three autopsied pancreata from patients with fulminant type 1 diabetes
| Morphometric analysis |
Frequency of insulitis |
Phenotype of MNCs infiltrating islets |
||||
|---|---|---|---|---|---|---|
| Islet volume (%) | β-Cell volume (%) | α-Cell volume (%) | [% (n1/n2)] | Macrophage (%) | CD8+ T-cell (%) | |
| Patient | ||||||
| Case 1 | 0.46 | 0.006 | 0.073 | 100 (34/34) | 42.3 | 38.4 |
| Case 2 | 0.94 | 0.129 | 0.350 | 100 (39/39) | 38.5 | 34.2 |
| Case 3 | 0.39 | 0.001 | 0.174 | 95 (21/22) | 70.2 | 24.6 |
| Mean value in patients | 0.60 ± 0.30* | 0.045 ± 0.073† | 0.199 ± 0.140 | 99 (94/95) | 50.3 ± 17.3 | 32.4 ± 7.1 |
| Mean value in nondiabetic control subjects (n = 10) | 3.14 ± 1.85 | 2.233 ± 1.431 | 0.300 ± 0.079 | 0 (0/747) | — | — |
Data are means ± SD, unless otherwise indicated. n1, number of the islets with insulitis; n2, number of the evaluated islets.
*P < 0.002;
†P < 0.001.
Predominant phenotypes of MNCs in islets with insulitis were macrophages and CD8+ T-cells (Fig. 2B and C) (Table 1). CD11c+ dendritic cells were detected in and around the islet with or without β-cells in cases 1 and 2 (Fig. 2D), while dendritic cell staining was less prominent in case 3. CD11c+ dendritic cell infiltration into islets was not observed in the 7 pancreatitis patients and 10 nondiabetic control subjects. B-lymphocytes, CD4+ T-cells, and NK cells were rare. VP1-positive pancreatic acinar cells were surrounded predominantly by macrophages (supplementary Fig. S3). In all three cases, MHC class II molecules were expressed on some residual β-cells (Fig. 2E). Macrophages did not show positive immunostaining for insulin (Fig. 2F), removing the possibility that macrophages with phagocytosed insulin vesicles from damaged β-cells represent MHC class II–expressing β-cells (27). Some vascular endothelium surrounding or inside the islets showed dilatation and enhanced expression of MHC class II molecules (Fig. 2G). MHC class I molecules were hyperexpressed on the pancreatic islet cells in three cases, while the islet cells of nondiabetic control pancreas showed only faint expression of MHC class I molecules in some islet cells (Fig. 2H and I). We could not detect interferon-α or 2′,5′-oligoadenylate synthetase–like protein on affected pancreata from patients with fulminant type 1 diabetes and control subjects, although these proteins represented markers of recent virus infection in pancreata affected by type 1 diabetes (28).
CXCL10 expressed in all islet cell subsets, which were infiltrated by CXCR3+ T-cells.
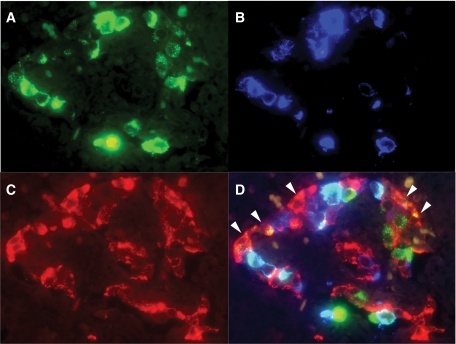
Double immunostaining demonstrated CXCL10 expression in pancreatic islets, while CXCR3-bearing MNCs that had infiltrated the islets expressed CXCL10 in all three cases (Fig. 2J). β-Cells, α-cells, and other subsets of islet cells expressed CXCL10 in all three cases (Fig. 3A–D). The positive cells for CXCL10 were observed in 96% (44 of 46), 100% (34 of 34), and 83% (31 of 38) of islets in case 1, case 2, and case 3, respectively. No CXCL10 expression was found in pancreatic acinar or ductal cells, which were surrounded by CD8+ T-cells and macrophages. Neither control pancreata nor that from the patient with slowly progressive type 1 diabetes expressed CXCL10 in the islets or exocrine pancreas (supplementary Figs. S4 and S5).
FIG. 3.
Triple-immnofluorescent staining for CXCL10 (A), insulin (B), and glucagon (C). A merged image (D) demonstrates expression of CXCL10 on β-cells (light blue) (case 2). A proportion of α-cells (orange, arrowheads) and other types of islet cells (green) also express CXCL10 (×400, case 2). (A high-quality digital representation of this figure is available in the online issue.)
Coexpression of CXCL10 and interferon-γ in islet cells.
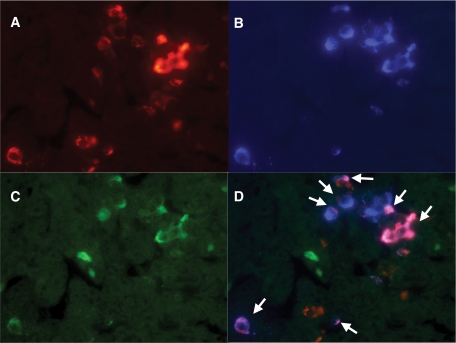
Interferon-γ was expressed in most β-cells, α-cells, and other types of islet cells from the cases with fulminant type 1 diabetes. Surprisingly, interferon-γ was coexpressed in CXCL10-positive islet cells (Fig. 4A–D). No CXCL10 or interferon-γ was expressed on affected exocrine pancreas or nondiabetic pancreas. RT-PCR could not show CXCL10 or interferon-γ sequences in the affected pancreas by fulminant type 1 diabetes. As we were unable to amplify 18S rRNA or GAPDH cDNAs from pancreatic sections of diabetic patients as mentioned above, we assumed that CXCL10 and interferon-γ RNAs had already degraded.
FIG. 4.
Triple-immunofluorescent staining for CXCL10 (A), insulin (B), and interferon-γ (C) in case 3. D: Merged image shows that residual β-cells express both CXCL10 and interferon-γ (arrows) (×400, case 3). (A high-quality digital representation of this figure is available in the online issue.)
Serum CXCL10 levels.
Serum CXCL10 levels in cases 1 and 2 were 563 pg/ml and 622 pg/ml, respectively. Serum CXCL10 levels were 13.5 times (case 1) and 15.0 times (case 2) higher than the mean value for healthy subjects (26).
DISCUSSION
We demonstrated various novel findings in fulminant type 1 diabetes that have not previously been reported for typical human type 1 diabetes (29–31). First, extensive enterovirus infection with severe infiltration of MNCs into both islets (insulitis) and the exocrine pancreas was observed around VP1-positive cells (Fig. 1A–C) (supplementary Fig. S3). Typical type 1 diabetic pancreas showed mild to moderate insulitis, distributed in a patchy manner throughout the pancreas (29,30), and VP1-positive cells could not be found in the exocrine pancreas (31). However, Richardson et al. (32) recently reported a high prevalence of VP1 in the islets of young patients with recent-onset type 1 diabetes using the same monoclonal antibody applied in our study. The VP1-positive pancreatic endocrine and exocrine cells showed characteristic features of cell damage, including shrunken and darkly stained nuclei suggestive of pyknosis, which was reported in Coxsackie virus–infected islets (31). Elevated serum pancreatic enzyme levels and pathological changes observed in virus-infected cells (Fig. 1C) (supplementary Fig. S3) showed enterovirus-associated involvement of the exocrine pancreas in this syndrome. Second, CXCL10 and interferon-γ were extensively coexpressed in islet cells (Fig. 4A–D). Most MNCs infiltrating into islets were either CD8+ T-cells bearing the CXCL10 receptor, CXCR3, or macrophages.
CXCL10 is a chemokine that is inducible by interferon-γ and exerts key roles in the expansion and attraction of autoreactive and antigen-specific T-cells (10,12). This finding of the coexpression of CXCL10 and interferon-γ in β-cells suggests the presence of a unique immunological circuit for accelerating β-cell destruction. The initial event that trigged CXCL10 expression on islet cells may be enteroviral infection of β-cells and the exocrine pancreas surrounding the islets (Fig. 1A–C). In vitro studies showed that enterovirus infection of islet cells induced CXCL10 production within 1–2 days after infection (33,34). In our patients, serum CXCL10 levels were elevated to >10 times higher than levels in control subjects. CXCL10 from islet cells will preferentially activate autoreactive T-cells via CXCR3 and thus attract cells to the islets releasing islet-specific antigen (10,12,13). The presence of activated autoreactive T-cells reacting with insulin B9-23 peptide, and GAD65 peptides has been reported in fulminant type 1 diabetes (35). Dendritic cells in the pancreas amplify immune responses to tissue antigens along with T-cells (36), thus contributing to rapid progression of β-cell failure. MHC class II molecules expressed on β-cells and dilated capillary endothelium around the islets (Fig. 2G) and aberrantly expressed MHC class II molecules on the islet cells will facilitate the “homing” process of activated T-cells and macrophages to islets (37–39). Our preliminary study examined the expression of another chemotactic protein besides CXCL10, namely monocyte chemotactic protein (MCP)-1. However, we failed to identify positive staining for MCP-1 on the affected pancreas, so CXCL10 and associated immunological cascades were studied. Chemoattracted autoreactive T-cells and macrophages brought to the islets will secrete interferon-γ and other inflammatory cytokines upregulating MHC class I molecules (Fig. 2H) and further destroy islet cells expressing CXCL10 (13,40). In such an extensively inflamed milieu in the islets, β-cells produce both CXCL10 and interferon-γ in the same cell (Fig. 4A–D). Interferon-γ in β-cells disturbs the function and viability of those cells and further accelerates CXCL10 generation and activation of autoreactive CXCR3-bearing T-cells and macrophages. These additional activated and accumulated T-cells and macrophages in the islets again secrete inflammatory cytokines including interferon-γ, inducing further CXCL10 generation in β-cells and CXCR3-mediated T-cell activation. This vicious cycle will continue until complete destruction of all β-cells has been achieved. The absence of expression of CXCL10 or interferon-γ in islets of the patient with slowly progressive type 1 diabetes (supplementary Fig. S5) supports the concept that CXCL10 and CXCR3 activation circuit represents a unique mechanism of rapid β-cell destruction in fulminant type1 diabetes.
Another unique finding in patients with fulminant type 1 diabetes was that both α- and β-cells in islets were infected by enterovirus and expressed CXCL10 and interferon-γ. In Coxsackie B4 enterovirus–induced type 1 diabetes, β-cells are specifically involved (41). Impaired α-cell volume was observed in case 1 and has been reported in long-standing patients with fulminant type 1 diabetes (42). Inflammatory processes were observed in pancreatic exocrine tissues in our study, and pancreatic enzyme is specifically increased in fulminant type 1 diabetes (3,4). These findings suggest that enterovirus-causing diabetes will display a wide diversity of tropism from β-cell-specific, as in cases of typical type 1 diabetes (41), to other subsets of pancreatic endocrine and exocrine cells, as in cases of fulminant type 1 diabetes. The genetic bondage of the host may have influence on virus potency or tropism. We have already reported specific genetic backgrounds (i.e., HLA-DRB1*0405 and HLA-DQB1*0401, which was possessed in our cases) for this syndrome (43).
The present findings regarding the destruction of islet endocrine cells provide new insights into strategies for the treatment of fulminant type 1 diabetes. Development of antagonists and neutralizing agents for interferon-γ and the CXCL10/CXCR3 axis may represent one therapeutic option. In an experimental animal model of type 1 diabetes, neutralization of CXCL10 can cure virus-induced type 1 diabetes (44).
Acknowledgments
This study was partly supported by grants from the Ministry of Education, Science, Sports, and Culture, Japan.
No potential conflicts of interest relevant to this article were reported.
Parts of this work were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 6–10 June 2009.
We thank T. Hughes for editorial assistance; Y. Kanemaru, C. Imai, and S. Takei for excellent secretarial work; and Drs. T. Momotsu and E. Okazaki from Niigata City General Hospital and Professor H. Fujii of the First Department of Surgery at the University of Yamanashi for their generous assistance with the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kobayashi T: Immunology and immunogenetics of type 1 diabetes in Japan. IDF Bulletin 1990;35:34–37 [Google Scholar]
- 2.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y: A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies: Osaka IDDM Study Group. N Engl J Med 2000;342:301–307 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka S, Kobayashi T, Momotsu T: A novel subtype of type 1 diabetes mellitus. N Engl J Med 2000;342:1835–1837 [PubMed] [Google Scholar]
- 4.Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Toyoda T, Maruyama T, Makino H: Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 2003;26:2345–2352 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Endo T, Aida K, Shimura H, Yokomori N, Kaneshige M, Furuya F, Amemiya S, Mochizuki M, Nakanishi K, Kobayashi T: Distinct diagnostic criteria of fulminant type 1 diabetes based on serum C-peptide response and HbA1c levels at onset. Diabetes Care 2004;27:1936–1941 [DOI] [PubMed] [Google Scholar]
- 6.Murase Y, Imagawa A, Hanafusa T, Iwahashi H, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H: Fulminant type 1 diabetes as a high risk group for diabetic microangiopathy: a nationwide 5-year-study in Japan. Diabetologia 2007;50:531–537 [DOI] [PubMed] [Google Scholar]
- 7.Hanafusa T, Imagawa A: Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab 2007;3:36–45 [DOI] [PubMed] [Google Scholar]
- 8.Charo IF, Ransohoff RM: The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610–621 [DOI] [PubMed] [Google Scholar]
- 9.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB: Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol 2003;171:6838–6845 [DOI] [PubMed] [Google Scholar]
- 10.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Holländer GA, Piali L: Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med 2002;8:1414–1420 [DOI] [PubMed] [Google Scholar]
- 11.von Herrath MG, Oldstone MB: Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J Exp Med 1997;185:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ejrnaes M, Videbaek N, Christen U, Cooke A, Michelsen BK, von Herrath M: Different diabetogenic potential of autoaggressive CD8+ clones associated with IFN-gamma-inducible protein 10 (CXC chemokine ligand 10) production but not cytokine expression, cytolytic activity, or homing characteristics. J Immunol 2005;174:2746–2755 [DOI] [PubMed] [Google Scholar]
- 13.Christen U, von Herrath MG: IP-10 and type 1 diabetes: a question of time and location. Autoimmunity 2004;37:273–282 [PubMed] [Google Scholar]
- 14.Nakagawa Y, Shimada A, Oikawa Y, Irie J, Shigihara T, Tsumura K, Narumi S, Saruta T: Two cases of “fulminant” type 1 diabetes suggesting involvement of autoimmunity. Ann N Y Acad Sci 2003;1005:359–361 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Tamemoto K, Nakanishi K, Kato N, Okubo M, Kajio H, Sugimoto T, Murase T, Kosaka K: Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care 1993;16:780–788 [DOI] [PubMed] [Google Scholar]
- 16.Oberste MS, Maher K, Flemister MR, Marchetti G, Kilpatrick DR, Pallansch MA: Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J Clin Microbiol 2000;38:1170–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nix WA, Berger MM, Oberste MS, Brooks BR, McKenna-Yasek DM, Brown RH, Jr, Roos RP, Pallansch MA: Failure to detect enterovirus in the spinal cord of ALS patients using a sensitive RT-PCR method. Neurology 2004;62:1372–1377 [DOI] [PubMed] [Google Scholar]
- 18.Nix WA, Oberste MS, Pallansch MA: Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 2006;44:2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lönnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, Savola K, Muona P, Simell T, Koskela P, Hyöty H: Enterovirus infection as a risk factor for β-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes 2000;49:1314–1318 [DOI] [PubMed] [Google Scholar]
- 20.Dominguez F, Martínez S, Quiñonero A, Loro F, Horcajadas JA, Pellicer A, Simón C: CXCL10 and IL-6 induce chemotaxis in human trophoblast cell lines. Mol Hum Reprod 2008;14:423–430 [DOI] [PubMed] [Google Scholar]
- 21.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM: Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 2008;118:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi K, Kobayashi T, Miyashita H, Okubo M, Sugimoto T, Murase T, Kosaka K, Hara M: Relationships among residual beta cells, exocrine pancreas, and islet cell antibodies in insulin-dependent diabetes mellitus. Metabolism 1993;42:196–203 [DOI] [PubMed] [Google Scholar]
- 23.Samuelson A, Forsgren M, Sällberg M: Characterization of the recognition site and diagnostic potential of an enterovirus group-reactive monoclonal antibody. Clin Diagn Lab Immunol 1995;2:385–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trabelsi A, Grattard F, Nejmeddine M, Aouni M, Bourlet T, Pozzetto B: Evaluation of an enterovirus group-specific anti-VP1 monoclonal antibody, 5-D8/1, in comparison with neutralization and PCR for rapid identification of enteroviruses in cell culture. J Clin Microbiol 1995;33:2454–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M, Kawata S, Tarui S, Kono N, Matsuzawa Y: Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993;92:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada A, Morimoto J, Kodama K, Suzuki R, Oikawa Y, Funae O, Kasuga A, Saruta T, Narumi S: Elevated serum IP-10 levels observed in type 1 diabetes. Diabetes Care 2001;24:510–515 [DOI] [PubMed] [Google Scholar]
- 27.In't Veld PA, Pipeleers DG: In situ analysis of pancreatic islets in rats developing diabetes. Appearance of nonendocrine cells with surface MHC class II antigens and cytoplasmic insulin immunoreactivity. J Clin Invest 1988;82:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark Å, Pujol-Borrell R, Rabinovitch A, Somoza N, Stewart TA: Interferon expression in the pancreases of patients with type I diabetes. Diabetes 1995;44:658–664 [DOI] [PubMed] [Google Scholar]
- 29.Lernmark Å, Klöppel G, Stenger D, Vathanaprida C, Fält K, Landin-Olsson M, Baskin DG, Palmer JP, Gown AM, Petersen JS: Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch 1995;425:631–640 [DOI] [PubMed] [Google Scholar]
- 30.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR: In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 [DOI] [PubMed] [Google Scholar]
- 31.Foulis AK, Farquharson MA, Cameron SO, McGill M, Schönke H, Kandolf R: A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 1990;33:290–298 [DOI] [PubMed] [Google Scholar]
- 32.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG: The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009;52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 33.Berg AK, Korsgren O, Frisk G: Induction of the chemokine interferon-gamma-inducible protein-10 in human pancreatic islets during enterovirus infection. Diabetologia 2006;49:2697–2703 [DOI] [PubMed] [Google Scholar]
- 34.Hultcrantz M, Hühn MH, Wolf M, Olsson A, Jacobson S, Williams BR, Korsgren O, Flodström-Tullberg M: Interferons induce an antiviral state in human pancreatic islet cells. Virology 2007;367:92–101 [DOI] [PubMed] [Google Scholar]
- 35.Kotani R, Nagata M, Imagawa A, Moriyama H, Yasuda H, Miyagawa J, Hanafusa T, Yokono K: T lymphocyte response against pancreatic beta cell antigens in fulminant type 1 diabetes. Diabetologia 2004;47:1285–1291 [DOI] [PubMed] [Google Scholar]
- 36.Melli K, Friedman RS, Martin AE, Finger EB, Miao G, Szot GL, Krummel MF, Tang Q: Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol 2009;182:2590–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pober JS, Cotran RS: Immunologic interactions of T lymphocytes with vascular endothelium. Adv Immunol 1991;50:261–302 [DOI] [PubMed] [Google Scholar]
- 38.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM: Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 2005;11:138–145 [DOI] [PubMed] [Google Scholar]
- 39.von Herrath M, Holz A: Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J Autoimmun 1997;10:231–238 [DOI] [PubMed] [Google Scholar]
- 40.Rabinovitch A, Suarez-Pinzon WL: Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 1998;55:1139–1149 [DOI] [PubMed] [Google Scholar]
- 41.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayama K, Imagawa A, Okita K, Uno S, Moriwaki M, Kozawa J, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I: Pancreatic beta and alpha cells are both decreased in patients with fulminant type 1 diabetes: a morphometrical assessment. Diabetologia 2005;48:1560–1564 [DOI] [PubMed] [Google Scholar]
- 43.Tanaka S, Kobayashi T, Nakanishi K, Koyama R, Okubo M, Murase T, Odawara M, Inoko H: Association of HLA-DQ genotype in autoantibody-negative and rapid-onset type 1 diabetes. Diabetes Care 2002;25:2302–2307 [DOI] [PubMed] [Google Scholar]
- 44.Ejrnaes M, von Herrath MG, Christen U: Cure of chronic viral infection and virus-induced type 1 diabetes by neutralizing antibodies. Clin Dev Immunol 2006;13:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]