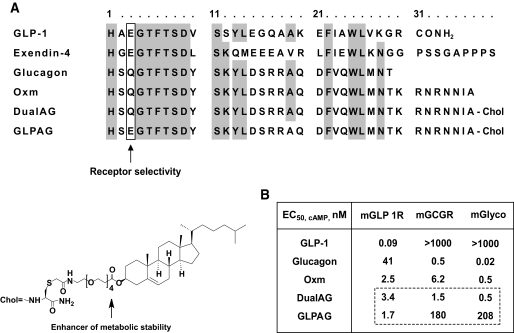
FIG. 1.
Sequence alignment and receptor agonist potencies of OXM and related peptides. A: Sequence alignment of OXM, exendin-4, and related glucagon superfamily peptides including the long acting OXM analogs DualAG and GLPAG. Conserved residues are highlighted. Gln3 is important for GCGR agonist activity. DualAG and GLPAG incorporate a DSer2 (S) substitution that confers resistance to DPP-4. The cholesterol moiety (chol) at the C-terminus of these peptides enhances metabolic stability and receptor affinity, and the intervening polyethylene glycol spacer minimizes the loss in agonist potency because of plasma protein/lipid binding. B: In vitro receptor agonist potencies (cAMP release) against mGLP1R and mGCGR and ED50 (nM) in the ex vivo mouse liver glycogenolysis assay (mGlyco). The Gln3→ Glu substitution in GLPAG reduces GCGR agonist activity ∼120- to 400-fold compared with DualAG.