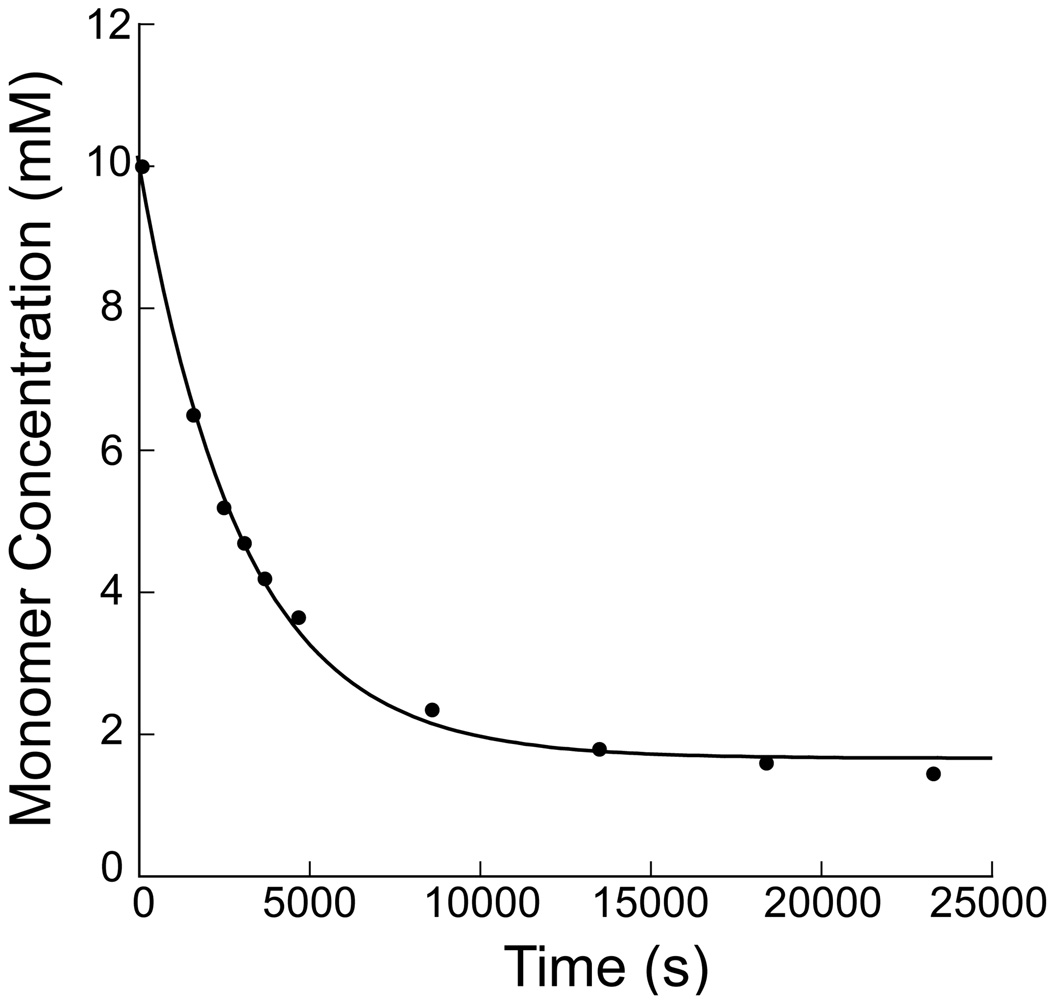
Figure 2.
The pseudo first order rate constant, kobs = 3 × 10−4 s−1 , for the reaction between monomer 2 (10 mM) and 3-amino-2-propanol (100 mM) was determined by monitoring the decrease in α-chloroacetamide methylene group resonance over time by NMR spectroscopy (see supporting information). The monomer concentration was calculated and plotted versus time (above). The kobs was determined using a first order decrease fit [y = A∞−((A∞−A0)*exp(−kobs*t)), where y = monomer concentration, t = time, A0 = initial concentration of 2, and A∞ = concentration of 2 at t∞).