Figure 13.
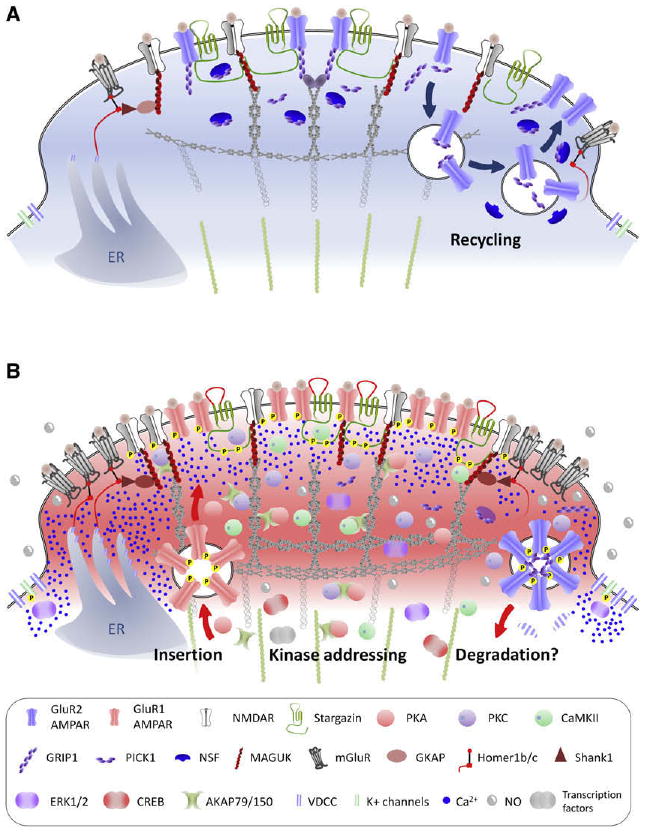
Role of scaffolding proteins in central sensitization. Representation of the post synaptic density (PSD) region of a synapse of a nociceptive neuron in the superficial lamina of the spinal cord under basal conditions (A) and during inflammatory pain (B). The proteins that make up the PSD can drive a major functional reorganization of synapses, modifying postsynaptic efficacy by altering receptor density at the membrane and producing switches from Ca2+-impermeable to Ca2+-permeable AMPARs. In addition, scaffolding proteins mediate the “addressing” of kinases to the receptors, thereby increasing their activity. Under normal conditions (A), neurons mostly express GluR2-containing AMPAR that are anchored to the PSD via GRIP-1. NMDAR are recycled in an activity-dependent manner via PICK-1 and NSF. During inflammation (B), there is an endocytosis of GluR2-containing AMPAR (initiated by PKC phosphorylation of GluR2) along with a loss of NSF that prevents their reinsertion into the synapse. GluR1-containing AMPAR are expressed at the membrane, where their activity is increased by phosphorylation with PKA as well as by the ectodomain of stargazin, whose C-terminus segment is phosphorylated by PKC and CaMKII. MAGUK can participate to increase glutamate receptors density at the synapse, and phosphorylated stargazin promotes AMPAR and NMDAR clustering. Scaffolding proteins such as AKAP79/150 and the MAGUKs also “address” kinases to specific synaptic position at the right time to phosphorylate AMPAR and NMDAR subunits, thereby increasing their activity. The Homer-Shank1-GKAP-MAGUK complex couples mGluR and NMDAR to intrasomal Ca2+ stores, so that activation of glutamate receptors leads to high levels of Ca2+ in the PSD that activate PKC and CaMKII, also recruited to the PSD by scaffolding proteins. PKC, CaMKII, and other kinases converge to activate ERK, which reduce K+ channels activity and promote transcriptional activity.
