Table 1.
(a) Palladium-Catalyzed Stereoselective Synthesis of N-Aryl-2-Benzylpyrrolidine Derivatives
| entry | amine | product | method | dr | yield(c) |
|---|---|---|---|---|---|
| 1 |

|
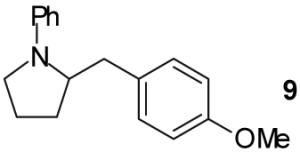
|
A | - | 67%(d) |
| 2 | B | - | 70%(d) | ||
| 3 | 1 |
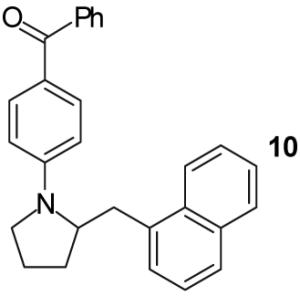
|
A | - | 72%(d) |
| 4 | B | - | 92%(d) | ||
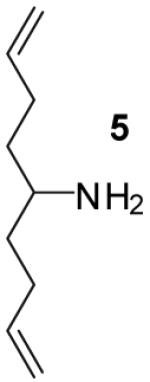
| 5 | 1 |
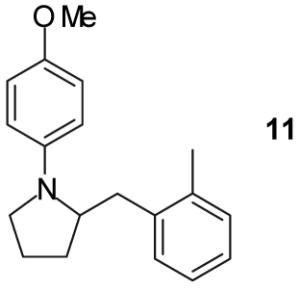
|
A | - | 17%(d) |
| 6 | B | - | 0% | ||
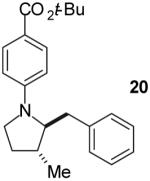
| 7 |
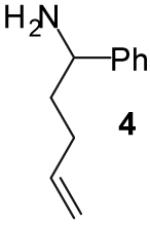
|
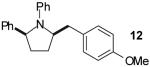
|
A | >20:1 | 68%(d) |
| 8 | 4 |
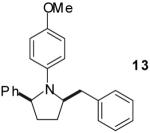
|
A | >20:1 | 67%(d) |
| 9 |
|
|
A | >20:1 | 70% |
| 10 | 5 |
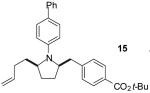
|
A | >20:1 | 65%(d) |
| 11 |
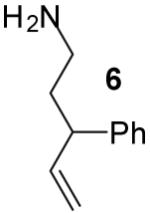
|
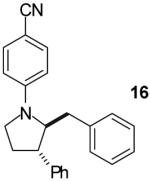
|
A | >20:1 | 56% |
| 12 | 6 |
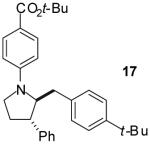
|
A | >20:1 | 41% |
| 13 | 6 |
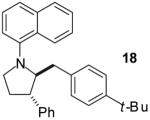
|
A | >20:1 | 51% |
| 14 |
|
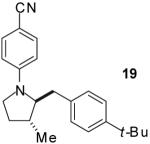
|
A | 9:1 | 71% |
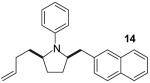
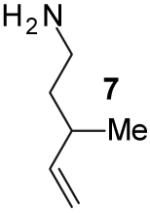
| 15 | 7 |
|
A | 9:1 | 42% |
| 16 |
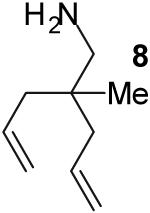
|
|
A | 3:2 | 49% |
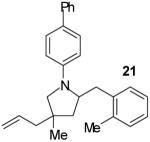
| 17 | 8 |
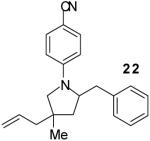
|
A | 3:2 | 63% |
Method A: 1.0 equiv amine, 1.0 equiv ArBr, 2.4 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % 3, toluene (0.125 M), 60 °C then 2 mol % dppe, 1.2 equiv Ar1Br, 110 °C. Method B: 1.0 equiv amine, 1.0 equiv ArBr, 2.4 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % (rac)-BINAP (2), toluene (0.125 M), 80 °C then 1.2 equiv Ar1Br, 80 °C.
Yields represent average isolated yields from two or more experiments.
This product contained a small amount (c.a. 5-10%) of an inseparable regioisomer.
