Table 2.
Scope of Ni-Catalyzed Enantioselective Dienal Allylationa
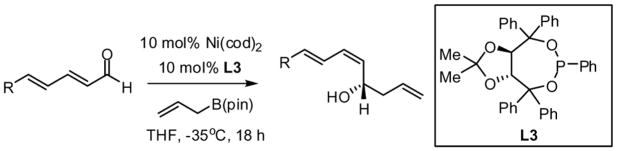 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | yield (%)b | (E,Z):(E:E) | %ee |
| 1 | 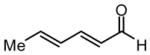 |
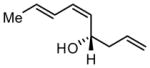 |
84 | >20:1 | 88 |
| 2 | 75 | 18:1 | 92 | ||
| 3 | 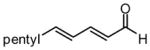 |
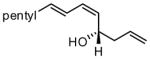 |
84 | >20:1 | 87 |
| 4 | 76 | >20:1 | 90 | ||
| 5 | 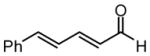 |
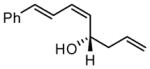 |
68 | >20:1 | 91 |
| 6 | 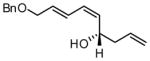 |
86 | 15:1 | 73 | |
| 7 | 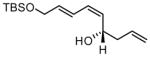 |
81 | 7:1 | 85 | |
| 8 | 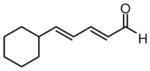 |
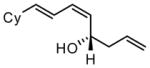 |
92 | >20:1 | 93 |
| 9 | 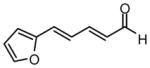 |
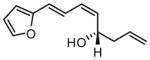 |
73 | 15:1 | 94 |
| 10 | 83 | 16:1 | 90 | ||
For entries 1,3, and 5–10: reaction carried out in a glovebox freezer. For entries 2 and 4, reaction carried out outside glovebox and quenched by addition of 30 equiv. of acetaldehyde, followed by warming to ambient. See the SI for details.
Isolated yield of purified material.
