Abstract
Objective
To investigate whether persons with treatment-resistant Lyme arthritis–associated HLA alleles might develop arthritis as a result of an autoimmune reaction triggered by Borrelia burgdorferi outer surface protein A (OspA), the Lyme disease vaccine antigen.
Methods
Persons in whom inflammatory arthritis had developed after Lyme disease vaccine (cases) were compared with 3 control groups: 1) inflammatory arthritis but not Lyme disease vaccine (arthritis controls), 2) Lyme disease vaccine but not inflammatory arthritis (vaccine controls), and 3) neither Lyme disease vaccine nor inflammatory arthritis (normal controls). HLA–DRB1 allele typing, Western blotting for Lyme antigen, and T cell reactivity testing were performed.
Results
Twenty-seven cases were matched with 162 controls (54 in each control group). Odds ratios (ORs) for the presence of 1 or 2 treatment-resistant Lyme arthritis alleles were 0.8 (95% confidence interval [95% CI] 0.3–2.1), 1.6 (95% CI 0.5–4.4), and 1.75 (95% CI 0.6–5.3) in cases versus arthritis controls, vaccine controls, and normal controls, respectively. There were no significant differences in the frequency of DRB1 alleles. T cell response to OspA was similar between cases and vaccine controls, as measured using the stimulation index (OR 1.6 [95% CI 0.5–5.1]) or change in uptake of tritiated thymidine (counts per minute) (OR 0.7 [95% CI 0.2–2.3]), but cases were less likely to have IgG antibodies to OspA (OR 0.3 [95% CI 0.1–0.8]). Cases were sampled closer to the time of vaccination (median 3.59 years versus 5.48 years), and fewer cases had received 3 doses of vaccine (37% versus 93%).
Conclusion
Treatment-resistant Lyme arthritis alleles were not found more commonly in persons who developed arthritis after Lyme disease vaccination, and immune responses to OspA were not significantly more common in arthritis cases. These results suggest that Lyme disease vaccine is not a major factor in the development of arthritis in these cases.
Lyme disease is a multisystem inflammatory illness caused by infection with the tick-borne spirochete Borrelia burgdorferi sensu stricto, transmitted by the bite of an infected Ixodes vector tick (1). Approximately 60% of patients with untreated Lyme disease who present with erythema migrans develop monarticular or oligoarticular arthritis. Lyme arthritis can be treated successfully with antibiotics; however, ~10% of Lyme arthritis patients will have persistent, chronic arthritis for months to several years despite antibiotic therapy, a condition referred to as treatment-resistant Lyme arthritis (1–7).
It has been suggested that an immune-mediated mechanism plays a role in the development of treatment-resistant Lyme arthritis (3–5). Both the presence of certain HLA–DRB1 alleles (primarily *0101, *0102, *0401, and *0404) and the level of serum IgG reactivity against the B burgdorferi surface antigen outer surface protein A (OspA) have been previously associated with treatment-resistant Lyme arthritis (4,8–11). Treatment-resistant Lyme arthritis has also been associated with T lymphocyte reactivity to OspA, and T cell cross-reactivity between an OspA epitope (OspA165–173) and the human lymphocyte function–associated antigen 1 epitope (hLFA1αL332–340) has been proposed as an autoimmune mechanism in the development of treatment-resistant Lyme arthritis, based on molecular mimicry arising from the high degree of sequence homology between the 2 epitopes (8).
The only Lyme disease vaccine licensed by the US Food and Drug Administration (FDA) (LYMErix; GlaxoSmithKline Pharmaceuticals, Research Triangle Park, NC/SmithKline Beecham Pharmaceuticals, Philadelphia, PA) contained recombinant OspA lipoprotein (rOspA) adsorbed to aluminum hydroxide adjuvant (12,13). The rOspA vaccine was shown to be safe and immunogenic in phase I and II trials, and a large randomized, double-blind, clinical trial in 5,469 vaccine recipients and 5,467 placebo recipients was conducted to assess safety and efficacy prior to licensure. The vaccine was found to be efficacious, and no statistically significant difference in the occurrence of arthritis between vaccine and placebo recipients was found during the clinical trial (12,13). The FDA licensed the Lyme disease vaccine on December 21, 1998 for the prevention of Lyme disease in individuals 15–70 years of age.
After licensure of the Lyme disease vaccine, the FDA and the Centers for Disease Control and Prevention (CDC) received reports through the Vaccine Adverse Event Reporting System (VAERS) of arthritis development in people who had received the vaccine. Review of these VAERS reports (14) revealed no clear pattern to suggest that the Lyme disease vaccine caused arthritis, although this conclusion was subject to the limitations of passive surveillance using spontaneous reports of adverse events (15). The manufacturer discontinued Lyme disease vaccine distribution on February 26, 2002, citing insufficient sales (16), and voluntarily withdrew the license several years later. Safety concerns remain regarding any OspA-based vaccine candidate (17,18).
Other possible mechanisms for the development of treatment-resistant Lyme arthritis have subsequently been suggested (19–23), but because this was the leading hypothesis at the time the vaccine was licensed, we sought to explore the possibility that people who developed arthritis after receiving the Lyme disease vaccine were more likely than controls to have treatment-resistant Lyme arthritis HLA alleles, or cellular and humoral immune responses to OspA. Such findings would support the hypothesis that the rOspA Lyme disease vaccine can cause inflammatory arthritis in genetically susceptible individuals.
METHODS
The VAERS was established in 1990 by the FDA and CDC as the passive surveillance system for reporting adverse events following immunization in the US. Reports are submitted by health care providers, vaccine recipients, vaccine manufacturers, and other interested parties. Passive surveillance systems such as the VAERS are subject to many limitations, including underreporting, incomplete information in many reports to make adequate diagnoses, inadequate data on the number of vaccine doses administered, and lack of a direct comparison group that does not introduce bias. These limitations usually make it difficult to determine if a vaccine caused the reported adverse event (15). However, the VAERS serves as a source of reports of possible cases of adverse events for investigation in case–control studies, through which many of the pitfalls of the VAERS can be avoided and understanding of the events improved.
To identify cases of new-onset inflammatory arthritis after Lyme disease vaccination, we searched data on the 1,048 adverse events after Lyme disease vaccination, reported between the date of licensure (December 21, 1998) and October 31, 2000, for reports containing the coding terms rheumatoid arthritis (RA), arthritis, arthrosis, joint disease, or arthralgia. Between December 2000 and May 2002, a telephone survey of patients reported to the VAERS (or of surrogate family member respondents) was conducted to obtain detailed information on clinical characteristics of the adverse event, patient demographic characteristics, Lyme disease vaccination, and medical history. Informed consent was obtained during the interview and by letter. There were up to 10 attempts to reach each reporter, health care provider, or patient.
Medical records were requested from the health care providers indicated by patients or family members during the interview. Medical records provided were abstracted, and 3 investigators (RB, SVS, and FWM [a rheumatologist]) conducted independent reviews to classify cases as definite or probable new-onset inflammatory arthritis, using standardized case definitions (Table 1). Differences in classification were resolved by discussion until consensus was reached. Cases were also evaluated to determine if they met the American College of Rheumatology (formerly, the American Rheumatism Association) 1987 revised criteria for the classification of RA (24) and reviewed to identify the most likely clinical diagnosis.
Table 1.
Criteria for classification of cases based on medical record documentation
| Classification as definite arthritis | |
| 1. | Affected joint(s) must show at least 3 of the 5 following signs of arthritis following vaccine administration: |
| a. Affected joint(s) painful at any time | |
| b. Limited motion of affected joint(s) | |
| c. Affected joint(s) tender (sensitive to touch) at any time | |
| d. Affected joint(s) warm at any time | |
| e. Swelling present in any of the affected joint(s) | |
| 2. | Duration of signs for at least 45 days following onset of first signs described in no. 1 |
| 3. | At least 1 joint continuously affected with joint effusion for at least 45 days, or at least 1 joint affected with intermittent effusion for at least 23 of 45 continuous days |
| Classification as probable arthritis | |
| 1. | Affected joint(s) must show at least 2 of the 5 following signs of arthritis following vaccine administration: |
| a. Affected joint(s) painful at any time | |
| b. Limited motion of affected joint(s) | |
| c. Affected joint(s) tender (sensitive to touch) at any time | |
| d. Affected joint(s) warm at any time | |
| e. Swelling present in any of the affected joint(s) | |
| 2. | Duration of signs for at least 30 days following onset of first signs described in no. 1 |
| 3. | At least 1 joint affected with intermittent effusion for at least 15 of 30 continuous days |
Individuals with definite new-onset inflammatory arthritis (cases) obtained from reports in the VAERS as described above were recruited to participate in a case–control study, and informed consent was obtained again. Three groups of age- (±5 years), sex-, and race-matched controls were established for the study. The arthritis control group consisted of patients who had not received the Lyme disease vaccine and who were treated for arthritis (no specified arthritis diagnosis excluded) of recent onset (within 24 months prior to enrollment). The vaccine control group consisted of people who had received the Lyme disease vaccine and did not report developing joint problems or arthropathy following vaccination. The normal control group consisted of people who had not received the Lyme disease vaccine and did not report experiencing any joint problems or arthropathy within 24 months prior to enrollment. A modified version of the case telephone survey was used to obtain information on control subjects’ demographic characteristics, Lyme disease vaccination, and medical history. For each case, 2 matched controls in each of the 3 control groups were recruited from a Lyme disease–endemic region (primarily Long Island, New York). Arthritis controls were recruited in collaboration with area rheumatologists. Vaccine controls were recruited from among individuals who had previously participated in a clinical trial of the Lyme disease vaccine. Normal controls were recruited from the community via advertisements. Subjects in these 3 control groups are also referred to below as epidemiologic controls. Subjects were compensated $50 for participating in the study, and health care providers were compensated $50 for collection and preparation of blood specimens from cases and controls.
Cases and controls donated 50 cc of peripheral whole blood for analysis. A 10-cc specimen was collected, using ACD sample collection tubes, for HLA allele testing, and a 40-cc specimen was collected, using sodium heparin sample collection tubes, for T cell reactivity and serologic testing. Prelabeled, prepaid shipping and packing materials and a unique, randomly generated study identification number were used to blind personnel at the collaborating laboratories with regard to the case–control status of each study sample.
DNA extraction for HLA allele typing was performed with an automated DNA prepping machine (GenoM6; Qiagen, Valencia, CA). Polymerase chain reaction (PCR) and sequence-based typing (SBT) reaction studies were performed with the GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). DRB1 generic SBT reaction analysis was performed using commercially available reagents (Abbott Molecular Diagnostics, Abbott Park, IL). Additionally, the generic typing was augmented for DRB1*01, *04, *12, and *14 alleles with locally synthesized sequencing primers (25). HLA–DRB1 PCR amplicons were used as a template for HLA SBT reactions. Exon 2 of HLA–DRB1 was bidirectionally sequenced utilizing the Sanger dideoxy sequencing method with BigDye Terminator V1.1 chemistry (Applied Biosystems). All sequencing data were collected with a 3100 Genetic Analyzer (Applied Biosystems). HLA allele assignments were interpreted from raw sequencing data using Assign SBT software (Conexio Genomics, Applecross, Western Australia, Australia).
Western blotting and serologic testing by enzyme-linked immunosorbent assay were conducted to evaluate subjects for serologic evidence of B burgdorferi infection and to measure specific antibody reactivity to OspA. Western blot analysis for IgG and IgM was performed using the Lyme Disease Marblot Strip Test System (MarDx, Carlsbad, CA). IgG Western blot results were considered positive for rOspA (+1, +2, or +3) if the 31-kd OspA band was present, and were otherwise considered negative. All tests were performed according to the manufacturer’s instructions. Western blot analyses were conducted, under blinded conditions with regard to case or control status, as specimens arrived at the laboratory, because the study protocol was to provide the test results to subjects as soon as possible, without waiting for accrual of matched samples. This policy did not allow us to use the same lot of reagents or conduct analyses on matched samples. To account for such variability in experimental conditions, all Western blot studies were repeated, again using the MarDx Lyme Disease Marblot Strip Test System under blinded conditions, at a separate international clinical reference laboratory for borrelioses at the National Center for Lyme Disease Reference and Research, CDC (http://www.cdc.gov/ncidod/dvbid/misc/bzb.htm). These repeat studies were performed using the same reagent lot, after all samples were collected.
Peripheral blood mononuclear cell (PBMC) T cell reactivity with OspA was tested by proliferation assay as previously described (4,19,26). Various concentrations of OspA peptide (1.11, 3.33, 10, or 30 µg/ml) or phytohemagglutinin (as a positive stimulation control) were used, with 3 wells used for each sample at each antigen concentration. Tritiated thymidine (0.5 µCi/well) was added for the last 18 hours of the assay. Tritiated thymidine uptake measured in scintillation counts per minute was measured in each well, and the mean cpm for each subject at each antigen concentration was calculated. Medium in the absence of any added antigen was used as a plate laboratory control. PBMCs from previously identified laboratory control subjects were used for internal assay controls; the positive internal laboratory control was a subject with confirmed Lyme disease, and the negative internal laboratory control was a subject with no known history of Lyme disease and no serologic evidence of exposure to B burgdorferi. The Δcpm was calculated for each individual by subtracting the cpm with medium alone from the cpm with antigen or mitogen stimulation, and the stimulation index (SI) was calculated by dividing the cpm with antigen or mitogen stimulation by the cpm with medium alone. Samples were grouped such that specimens from each case subject and the 6 controls matched to that case were tested on the same plate assay to control for interassay variation; however, laboratory investigators were blinded with regard to the case or epidemiologic control status of each subject. Results were read as positive or negative.
Odds ratios (ORs) for the presence of 1 treatment-resistant Lyme arthritis HLA allele (HLA–DRB1*0101, *0102, *0401, or *0404) and for the presence of 2 of these alleles, comparing cases with each control group (arthritis control, vaccine control, normal control), were calculated using Mantel-Haenszel stratification to account for matching. ORs for positive serologic response to OspA and for positive PBMC T cell reactivity with OspA were also calculated comparing cases and vaccine epidemiologic controls. Ninety-five percent confidence intervals (95% CIs) were calculated using exact methods and Stata software, version 8.0 (StataCorp, College Station, TX). To account for number of doses of vaccine received, time since vaccination, and presence of a treatment-resistant Lyme arthritis allele, conditional logistic regression (or exploratory standard logistic regression if conditional logistic regression analyses failed to converge) was used to analyze the immune response to the vaccine in cases and vaccine controls. Exploratory analyses (not prespecified) of the proportions of HLA alleles and IgG antibodies in the case and control groups were also conducted using Fisher’s 2-sided exact testing for statistical significance. Case–control comparisons were made using both sets of Western blot results, and consistency between results assessed. The level of agreement between measurements of T cell response (Δcpm and SI) was calculated using the kappa statistic.
The study was approved by the FDA Research Involving Human Subjects Committee and the institutional review boards of the Biomedical Research Alliance of New York, Johns Hopkins University, and Tufts–New England Medical Center.
RESULTS
There were 406 reports to the VAERS between December 21, 1998 and October 31, 2000 that contained at least 1 of the coding terms: rheumatoid arthritis (19 reports), arthritis (70 reports), arthrosis (45 reports), joint disease (32 reports), and arthralgia (240 reports). Ten were excluded because the subject was <18 years old. Contact information was available for 194 reporters, and 155 reporters were reached. Eighteen subjects refused to participate, and we completed interviews with 137 reporters. One hundred eleven subjects returned the release forms, and 150 health care providers submitted records on 90 of these 111 subjects. With the addition of medical records previously submitted to the VAERS on 28 other subjects, at least partial medical records for 118 interview subjects were available and were evaluated in the present study. We identified 34 cases for which there was health care provider documentation of new-onset inflammatory arthritis following Lyme disease vaccine (30 definite and 4 probable cases). We recruited into the case–control study 27 of the 30 identified cases of new-onset definite inflammatory arthritis (90%). We also recruited 2 age- (±5 years), sex-, and race-matched controls for each case into each of the 3 control groups, for a total of 162 control subjects (54 arthritis controls, 54 vaccine controls, and 54 normal controls).
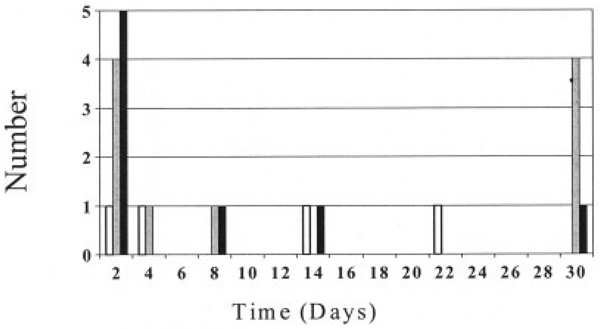
Demographic and clinical characteristics of the study subjects are shown in Table 2. A higher percentage of cases (59%) than arthritis controls (46%) had a reported diagnosis of RA. Cases were from Virginia, Maryland, New Jersey, New York, Pennsylvania, Rhode Island, Connecticut, Massachusetts, Minnesota, Illinois, Iowa, and Florida. All but 1 of the controls were from New York. Among cases there was no clear clustering of arthritis onset by vaccine dose or time since vaccination. The relationship between time to onset of arthritis symptoms and dose of Lyme disease vaccine in cases of new-onset definite inflammatory arthritis is detailed in Figure 1. In 23 of the 27 patients, inflammatory arthritis occurred within 30 days after administration of dose 1, 2, or 3 of the vaccine.
Table 2.
Demographic and clinical characteristics of the cases and controls*
| Cases (n = 27) |
Arthritis controls (n = 54) |
Vaccine controls (n = 54) |
Normal controls (n = 54) |
|
|---|---|---|---|---|
| Age, mean (range) years | 55.0 (41–74) | 54.4 (37–77) | 55.0 (39–74) | 54.3 (38–74) |
| Female sex | 16 (59) | 32 (59) | 32 (59) | 32 (59) |
| Total no. of Lyme vaccine doses, no. receiving 1/2/3 | 3/14/10 | NA | 0/4/50 | NA |
| Time since last dose, median (range) years | 3.59 (2.75–5.09) | NA | 5.48 (2.75–8.60) | NA |
| Met ACR RA criteria | 14 (52) | 19 (35) | NA | NA |
| Diagnosis of RA | 16 (59) | 25 (46) | NA | NA |
| Other arthritis diagnoses | 11 (41)† | 29 (54)‡ | NA | NA |
Except where indicated otherwise, values are the number (%). NA = not applicable; ACR = American College of Rheumatology; RA = rheumatoid arthritis.
Eight (30%) undifferentiated arthritis; 2 (7%) osteoarthritis; 1 (4%) arthritis and scleroderma.
Seventeen (31%) undifferentiated arthritis; 6 (11%) psoriatic arthritis; 3 (6%) gouty arthritis; 1 (2%) osteoarthritis; 1 (2%) Crohn’s disease–related arthritis; 1 (2%) parvovirus-related arthritis.
Figure 1.
Time from administration of last dose of Lyme disease vaccine to onset of arthritis. Doses 1, 2, and 3 are represented by open bars, shaded bars, and solid bars, respectively. Four additional subjects developed arthritis but are not included in the graph (1 who developed arthritis 60 days after dose 3 [90 days after dose 2], 2 who developed arthritis 210 days after dose 2, and 1 who developed arthritis after dose 2 [number of days after dose unknown]).
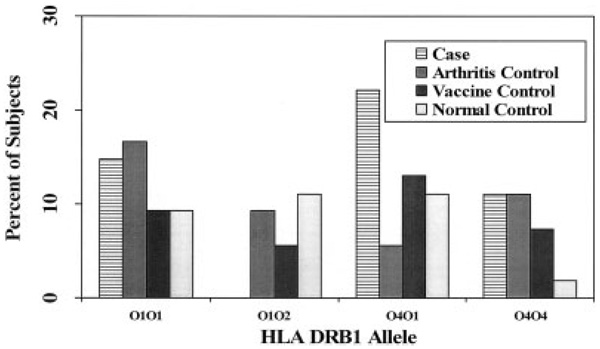
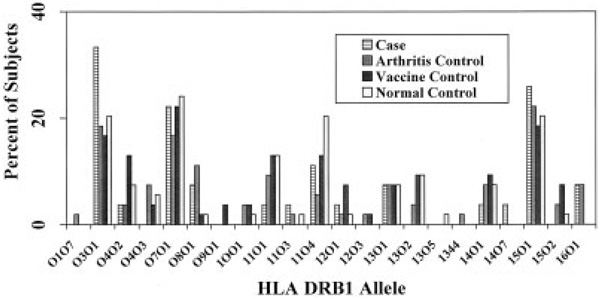
The prevalence of positivity for 1 or 2 treatment-resistant Lyme arthritis alleles in cases and controls, with ORs and 95% CIs, is shown in Table 3. An exploratory comparison of the prevalence of each DRB1 allele between cases and controls revealed no significant differences at the α = 0.05 level, although in the comparison of the prevalence of the *0401 allele between cases and arthritis controls, the difference approached significance (P = 0.054). The proportions of cases and controls with treatment-resistant Lyme arthritis HLA–DRB1 alleles are shown in Figure 2, and the proportions with DRB1 alleles not associated with treatment-resistant Lyme arthritis are shown in Figure 3.
Table 3.
Prevalence of treatment-resistant Lyme arthritis HLA–DRB1 alleles and odds ratio between cases and each of the 3 control groups*
| Cases (n = 27) |
Arthritis controls (n = 54) |
Vaccine controls (n = 54) |
Normal controls (n = 54) |
|
|---|---|---|---|---|
| 1 or 2 TRLA alleles | ||||
| Prevalence, no. (%) | 15 (56) | 31 (57) | 28 (52) | 29 (54) |
| Odds ratio (95% CI) | – | 0.8 (0.3–2.1) | 1.6 (0.5–4.4) | 1.75 (0.6–5.3) |
| 2 TRLA alleles | ||||
| Prevalence, no. (%) | 4 (15) | 3 (5.6) | 3 (5.6) | 4 (7.4) |
| Odds ratio (95% CI) | – | 3.5 (0.6–21.5) | 2.7 (0.6–11.9) | 2.3 (0.5–11.6) |
Treatment-resistant Lyme arthritis (TRLA) alleles are *0101, *0102, *0401, and *0404. 95% CI = 95% confidence interval.
Figure 2.
Percentage of cases and controls with 1 or 2 treatment-resistant Lyme arthritis–associated HLA–DRB1 alleles. There were no statistically significant differences between groups. For the percentage of DRB1*0401-positive cases versus the percentage of DRB1*0401-positive arthritis controls, the difference approached nominal statistical significance (P = 0.054).
Figure 3.
Percentage of cases and controls with 1 or 2 HLA–DRB1 alleles not associated with treatment-resistant Lyme arthritis. There were no statistically significant differences between groups.
In the first Western blot analysis, the prevalence of antibodies to OspA in cases, arthritis controls, vaccine controls, and normal controls was 52%, 2%, 81%, and 2%, respectively. The prevalences were similar in the independent repeat blinded Western blot analysis of these sera at the CDC reference laboratory (33%, 7%, 81%, and 0%, respectively). Cases were less likely than vaccine controls to have IgG antibodies to OspA in the original Western blot analysis (OR 0.3 [95% CI 0.1–0.8]) and the repeat Western blot analysis (OR 0.13 [95% CI 0.04–0.44]). Cases were also less likely than vaccine controls to have received 3 doses of vaccine (37% versus 93%), and the samples were obtained a median of 3.59 years after vaccination (range 2.75–5.09) in cases and a median of 5.48 years after vaccination (range 2.75–8.6) in vaccine controls. Conditional logistic regression could not be used to adjust for differences in number of doses received, time since vaccination, and presence of a treatment-resistant Lyme arthritis allele, because some substrata contained 0 pairs. Exploratory logistic regression revealed that only the number of doses was a statistically significant factor, and after adjustment for the number of doses received, cases were still not significantly more likely than vaccine controls to have an OspA IgG antibody band (OR 1.6 [95% CI 0.3–8.6]).
An initial exploratory comparison of IgG bands other than OspA showed significant differences between cases and all control groups for antibodies to the 41-kd antigen and to the 58-kd antigen. However, these differences were not confirmed in the independent repeat blinded Western blot analysis of these sera at the CDC reference laboratory.
PBMC T cell reactivity to OspA, assessed by proliferation assay, was evaluated using the SI and Δcpm in all cases and controls. The SI was positive in 7 cases (26%), 6 arthritis controls (11%), 10 vaccine controls (19%), and 6 normal controls (11%). The odds of a positive T cell response after stimulation with OspA antigen, measured by SI, were not significantly different between cases and vaccine controls (OR 1.6 [95% CI 0.5–5.1]). Similarly, T cell stimulation assays in which the response was measured by Δcpm showed no significant difference between cases and controls (OR 0.7 [95% CI 0.2–2.3]). Adjustment by conditional logistic regression for number of doses of vaccine received, time since vaccination, and presence of a treatment-resistant Lyme arthritis allele did not materially alter these findings. Among cases and vaccine controls, the kappa statistic for agreement between the SI and Δcpm measures of T cell response was 0.3, indicating a low level of agreement.
DISCUSSION
This study was undertaken to explore the possibility that people who have developed arthritis after receiving the Lyme disease vaccine are more likely than controls to have certain arthritis-associated HLA alleles or immune responses to the vaccine. Such findings would support the hypothesis that the Lyme disease vaccine can cause inflammatory arthritis in genetically susceptible individuals. Neither treatment-resistant Lyme arthritis HLA–DRB1 alleles nor any other HLA alleles we tested were found with significantly greater frequency in people who developed arthritis after receiving the Lyme disease vaccine. In addition, neither antibody nor T cell responses to OspA were significantly more common in arthritis cases, suggesting that cross-reactivity between OspA and a human antigen is unlikely to be a factor underlying the arthritis in any substantial proportion of the cases. Furthermore, as shown in Figure 1, the short duration between last vaccine dose and onset of arthritis in many cases makes an immunopathogenetic linkage by commonly accepted mechanisms unlikely.
The treatment-resistant Lyme arthritis HLA–DRB1 alleles *0101, *0102, *0401, and *0404 share an epitope in the third hypervariable region with other HLA alleles that have been associated with RA (27), and these alleles would therefore likely be more common in patients with RA. Nevertheless, if the Lyme disease vaccine preferentially caused arthritis in people with treatment-resistant Lyme arthritis HLA alleles, one would expect that significantly more cases than arthritis controls would have been positive for these alleles in our study. However, this finding was not observed.
Although cases were more likely than controls to have 2 treatment-resistant Lyme arthritis alleles, several factors, in addition to the lack of statistical significance of the association, make it likely that this result was an artifact. First, one would expect a higher percentage of cases with treatment-resistant Lyme arthritis alleles compared with vaccine controls or normal controls, since treatment-resistant Lyme arthritis alleles are also associated with RA and would likely be more common in any group that includes patients with RA. Second, most studies demonstrating HLA associations with autoimmune diseases have shown links with a single copy of a given allele, and in only a few diseases does homozygosity further increase the risk (28); it would be very unusual to find an increased risk of an autoimmune illness associated with the presence of 2 suspect alleles but not to find any increase when just 1 suspect allele is present. Third, this analysis was not prespecified, making it susceptible to the increased likelihood of chance associations inherent in exploratory analyses. Finally, since only a small proportion of cases had 2 treatment-resistant Lyme arthritis HLA alleles, this association could not explain the vast majority of arthritis adverse events we studied.
In the exploratory comparison of cases versus arthritis controls, the frequency of positivity for 1 treatment-resistant Lyme arthritis allele, *0401, approached nominal statistical significance (P = 0.054). However, this result is likely explained by the lower-than-expected prevalence of this allele in the arthritis controls, even compared with both the normal and vaccine controls. Furthermore, the potential for bias in reporting with regard to DR4 alleles due to publicity surrounding the hypothesis of adverse events possibly occurring in people with these alleles, the low proportion of cases with the *0401 allele, the lack of adjustment of the alpha level for the number of comparisons in the exploratory analysis, and the absence of a similar finding for other treatment-resistant Lyme arthritis alleles with a shared epitope in the third hypervariable region make this less likely to be a true association.
With regard to immune response, the comparison of most interest is between cases and vaccine controls. If the Lyme disease vaccine were to cause arthritis by means of a cross-reactive immune response between OspA and some human antigen, one could hypothesize that the OspA antibody and T cell response would be more common in arthritis cases than vaccine controls. This, however, was not observed. Indeed, based on the magnitude of the effect reported from the study advancing the molecular mimicry hypothesis for treatment-resistant Lyme arthritis (8), we would have expected, but did not find, a strong immune response to OspA among the cases if the vaccine was causing arthritis by this mechanism. A major limitation of this study is the fact that several years passed between vaccination and analysis of the immune response. These responses decline rather rapidly after vaccination and may not be accurately reflected in samples tested several years later. Statistically significant associations in the exploratory comparison of IgG bands other than OspA (41-kd and 58-kd antigens) between cases and controls from the first Western blot analysis were not confirmed with analysis of the repeat Western blots in the international clinical reference laboratory for borrelioses, using the same reagent lot. We believe the findings of these repeat analyses are more likely to be correct and suggest that the initial results were likely due to interassay variation and not to a true difference between cases and controls.
Although previous findings suggesting possible molecular mimicry between OspA165–173 and hLFA1αL332–340 were the impetus for this study, our results are consistent with more recent findings showing minimal T cell responses to hLFA1αL332–340 in patients with treatment-resistant Lyme arthritis (19–21). These latter findings have cast doubt on the earlier hypothesis and raised other possibilities. One alternative is that T cell responses to OspA165–173 or other B burgdorferi epitopes in genetically susceptible individuals may be particularly inflammatory, which could cause bystander activation of autoreactive T cells to induce and maintain arthritis after apparent spirochetal killing (22). This is supported by the finding that, compared with patients with antibiotic-responsive arthritis, those with treatment-resistant Lyme arthritis had significantly higher synovial fluid levels of Th1 cytokines, which increased over time (23).
A potential limitation of this study, in addition to the time elapsed between vaccination and measurement of the immune response as noted above, is the fact that cases and vaccine controls were not matched for the number of doses of vaccine received. It is known from the clinical trial that the immune response, including the magnitude of the T cell proliferative response, should be greater after a larger number of doses and shorter time interval since vaccination. Statistical analysis incorporating these 2 factors in our study revealed that only the number of doses of vaccine received had a statistically significant effect, and the effect was only on the OspA antibody response. Moreover, taking the number of doses into account did not materially alter the conclusions from the unadjusted analysis, and thus the lack of matching for number of doses received and time elapsed since vaccination is unlikely to have substantially affected our results. A further limitation is that, while treatment-resistant Lyme arthritis is thought to be initiated by natural infection with a complex organism against which the body mounts a vigorous immune response, the vaccine is composed of a single recombinant protein and it is challenging to observe the immune response to this protein, especially a long time after receipt of the last vaccine dose. Finally, although we attempted to contact and enroll every possible subject with arthritis reported to the VAERS, the small number of cases limited the power of this study to detect small effects, and selection of all cases from the VAERS may limit generalizability of the results.
This is likely to be the only immunogenetic study of arthritis development after Lyme disease vaccination, because the vaccine is no longer marketed, >6 years have elapsed since the vaccine was widely used, and this adverse event is rare. These practical and scientific difficulties would be virtually insurmountable in implementing another such study in the future. Despite the limitations of the present study, the findings are reassuring with respect to the vaccine’s safety relative to arthritis. In future investigations of other OspA-based Lyme disease vaccines, a similar study design for rigorously assessing hypothesized arthritis risks should be considered.
ACKNOWLEDGMENTS
We are grateful to Jane Woo, Pat Lesho, and personnel of the Battelle Memorial Institute (Columbus, OH) for assistance with interviews.
Supported by the US Department of Health and Human Services National Vaccine Program Office, the international program of the National Institute of Environmental Health Sciences, NIH, and the FDA. Dr. Shadomy is recipient of a fellowship from the US Department of Health and Human Services National Vaccine Program Office.
Dr. Meyer is coinventor on “Borrelia burgdorferi polypeptides and uses thereof” (US patent 6,689,364) and worked from December 2003 through December 2004 on a Sponsored Research Project with Baxter/AG examining their potential Lyme disease vaccine proteins in model systems. Drs. Leffell and Zachary owned stock in the Luminex and Qiagen companies, which generate reagents used in the laboratory for HLA typing. The Biomedical Research Alliance of New York was funded by GlaxoSmithKline to serve as a site for the clinical trial of the Lyme disease vaccine from 1994 to 1998, but received no subsequent Lyme disease vaccine–related funding from GlaxoSmithKline during the conduct of this study.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Ball had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Ball, Shadomy, Meyer, Huber, Leffell, Zachary, Miller, Braun.
Acquisition of data. Ball, Shadomy, Meyer, Huber, Leffell, Zachary, Belotto, Hilton, Schriefer, Miller.
Analysis and interpretation of data. Ball, Shadomy, Meyer, Huber, Leffell, Zachary, Schriefer, Miller, Braun.
Manuscript preparation. Ball, Shadomy, Meyer, Hilton, Schriefer, Miller, Braun.
Statistical analysis. Ball, Shadomy, Genevier.
REFERENCES
- 1.Steere AC. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH, III, Liu NY, et al. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC. Pathogenesis of Lyme arthritis: implications for rheumatic disease. Ann N Y Acad Sci. 1988;539:87–92. doi: 10.1111/j.1749-6632.1988.tb31841.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Field JA, Glickstein L, Molloy PJ, Huber BT, Steere AC. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum. 1999;42:1813–1822. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Kamradt T, Krause A, Burmester GR. A role for T cells in the pathogenesis of treatment-resistant Lyme arthritis. Mol Med. 1995;1:486–490. [PMC free article] [PubMed] [Google Scholar]
- 6.Burmester GR, Daser A, Kamradt T, Krause A, Mitchison NA, Sieper J, et al. Immunology of reactive arthritides. Annu Rev Immunol. 1995;13:229–250. doi: 10.1146/annurev.iy.13.040195.001305. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis [review] Arthritis Rheum. 2006;54:3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 8.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 9.Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA–DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 11.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–971. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lymerix [package insert] Philadelphia (PA): SmithKline Beecham Pharmaceuticals; 2000. [Google Scholar]
- 13.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, et al. for the Lyme Disease Vaccine Study Group. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 14.Lathrop S, Ball R, Haber P, Mootrey GT, Braun MM, Shadomy SV, et al. Adverse event reports following vaccination for Lyme disease: December 1998-July 2000. Vaccine. 2002;20:1603–1608. doi: 10.1016/s0264-410x(01)00500-x. [DOI] [PubMed] [Google Scholar]
- 15.Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287–294. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hitt E. Poor sales trigger vaccine withdrawal. Nat Med. 2002;8:311–312. doi: 10.1038/nm0402-311b. [DOI] [PubMed] [Google Scholar]
- 17.Willett TA, Meyer AL, Brown EL, Huber BT. An effective second-generation outer surface protein A-derived Lyme vaccine that eliminates potentially autoreactive T cell epitope. Proc Natl Acad Sci U S A. 2004;101:1303–1308. doi: 10.1073/pnas.0305680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott A. Lyme disease: uphill struggle. Nature. 2006;439:524–525. doi: 10.1038/439524a. [DOI] [PubMed] [Google Scholar]
- 19.Trollmo C, Meyer AL, Steere AC, Hafler DA, Huber BT. Molecular mimicry in Lyme arthritis demonstrated at the single cell level: LFA-1 is a partial agonist for outer surface protein reactive T cells. J Immunol. 2001;166:5286–5291. doi: 10.4049/jimmunol.166.8.5286. [DOI] [PubMed] [Google Scholar]
- 20.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Searching for Borrelial T cell epitopes associated with antibiotic-refractory Lyme arthritis [published erratum appears in Mol Immunol 2008;45:3508] Mol Immunol. 2008;45:2323–2332. doi: 10.1016/j.molimm.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannian P, Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Decline in the frequencies of Borrelia burgdorferi OspA161 175-specific T cells after antibiotic therapy in HLA-DRB1* 0401-positive patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. J Immunol. 2007;179:6336–6342. doi: 10.4049/jimmunol.179.9.6336. [DOI] [PubMed] [Google Scholar]
- 22.Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol. 2001;2:797–801. doi: 10.1038/ni0901-797. [DOI] [PubMed] [Google Scholar]
- 23.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Kotsch K, Wehling J, Blasczk R. Sequencing of HLA class II genes based on the conserved diversity of the non-coding regions: sequencing based typing of HLA-DRB genes. Tissue Antigens. 1999;53:486–497. doi: 10.1034/j.1399-0039.1999.530505.x. [DOI] [PubMed] [Google Scholar]
- 26.Meyer AL, Trollmo C, Crawford F, Marrack P, Steere AC, Huber BT, et al. Direct enumeration of Borrelia-reactive CD4 T cells ex vivo by using MHC class II tetramers. Proc Natl Acad Sci U S A. 2000;97:11433–11438. doi: 10.1073/pnas.190335897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorman JD, David-Vaudy E, Pai M, Lum RF, Criswell LA. Particular HLA–DRB1 shared epitope genotypes are strongly associated with rheumatoid vasculitis. Arthritis Rheum. 2004;50:3476–3484. doi: 10.1002/art.20588. [DOI] [PubMed] [Google Scholar]
- 28.Caruso C, Candore G, Colonna-Romano G, Lio D, Bonafe M, Valensin S, et al. HLA, aging, and longevity: a critical reappraisal. Hum Immunol. 2000;61:942–949. doi: 10.1016/s0198-8859(00)00168-3. [DOI] [PubMed] [Google Scholar]