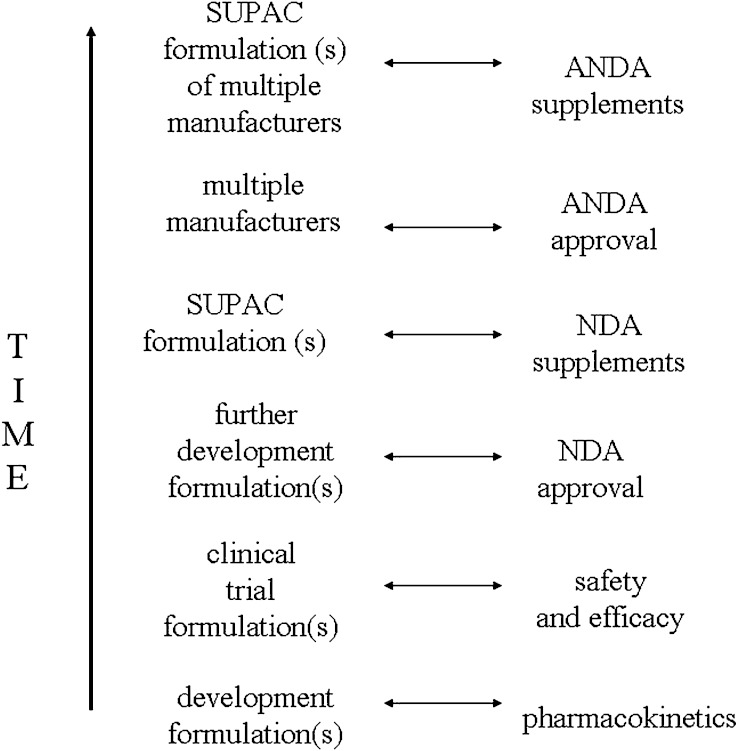
Fig. 1.
Formulations in the normal course of a drug product’s life cycle. Even shortly after marketing of a new drug, the marketed formulation differ from the clinical trial formulation that demonstrated drug safety and efficacy, due to formulation changes in later development and SUPAC changes. In later stages of drug market life, formulations from several generic manufacturers are available, including generic formulations with SUPAC changes