Abstract
The biopharmaceutical classification system (BCS) classifies compounds based on their solubility and permeability. Regulatory agencies and health organizations have utilized this classification system to allow dissolution to be used to establish bioequivalence for highly soluble and highly permeable compounds. The pharmaceutical industry has taken advantage of this and BCS-based waivers are becoming more routine and result in significant savings. Further, there is strong scientific rationale to allow BCS-based waivers for even more compounds to realize even more savings. Yet just as clear as the benefits are the barriers that limit application: lack of international regulatory harmonization, uncertainty in regulatory approval, and organizational barriers within the pharmaceutical industry. Once these barriers are overcome and additional applications are fully allowed, the full benefits of BCS applications will be realized.
Key words: biopharmaceutical classification system, dissolution, formulations development, permeability, solubility
INTRODUCTION
Amidon et al. first proposed a biopharmaceutic classification system (BCS) in 1995, that classified compounds based on their solubility and permeability (1). Subsequently, regulatory agencies and health organizations have utilized this classification system to allow in vitro dissolution to be used to establish bioequivalence for highly soluble and highly permeable compounds (2). The pharmaceutical industry has taken advantage of dissolution and BCS-based waivers of in vivo studies; however, this is not the only instance where application of the BCS is beneficial. Rather, its principles are used throughout development. A series of case studies are presented to illustrate uses of BCS through out the clinical development cycle followed by a discussion of its implementation in that aspect of clinical development.
CASE STUDIES I-II—APPLICATION OF BCS IN INNOVATOR DRUG DEVELOPMENT
Case Study I—Use of the BCS in Formulation Development
Pregabalin (Lyrica®) is described chemically as (S)-3-(aminomethyl)-5-methylhexanoic acid. It binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin is unknown, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin’s antinociceptive and antiseizure effects in animal models. In vitro, pregabalin reduces the calcium dependent release of several neurotransmitters, possibly by modulation of calcium channel function. Pregabalin is indicated for the management of neuropathic pain associated with diabetic peripheral neuropathy, management of postherpetic neuralgia, adjunctive therapy for adult patients with partial onset seizures, and management of fibromyalgia (3).
Pregabalin is a BCS Class 1 compound (highly permeable and highly soluble). Pregabalin is an amino acid and its lowest aqueous solubility occurs at its isoelectric point (at pH 7.4). It is considered high solubility as the amount of water needed (<10 mL) to dissolve the highest dose strength (300 mg) at pH 7.4 is less than the 250 mL criteria. Pregabalin meets the BCS criteria for a highly permeable compound as greater than 90% of the dose is excreted unchanged in the urine (4).
Three different series of formulations were used during clinical development. Each series was comprised of one to three different dose strengths. Strengths within each series were content proportional with respect to drug and excipients. All three series used the same excipients; however the relative proportion of each excipient was different for each series. Bioequivalence between and among these formulations and the commercial formulation was established for this BCS Class 1 compound by demonstrating that all formulations were rapidly dissolving and had similar dissolution profiles over a pH range of 1.2 to 6.8. Thus bioequivalence was demonstrated using dissolution data and waivers of in vivo bioequivalence studies were granted (4,5).
Discussion—Use of the BCS in Formulation Development
Application of BCS had significant impact on the cost of clinical development program for pregabalin. It has been estimated that this example saved the company more than $1,000,000 compared to a more traditional approach that would have utilized four separate bioequivalence studies. Further, indirect savings are equally impressive. Considering that yearly Pregabalin sales exceed $1,200,000,000, each month of times saving equates to an additional $100,000,000 in sales prior to loss of patent exclusivity. Looking across the pharmaceutical industry, approximately 30% of all oral immediate release drugs can be classified as highly permeable and highly soluble (6). It has been estimated that the pharmaceutical industry can save over $35,000,000/year in direct savings through application of the BCS (7).
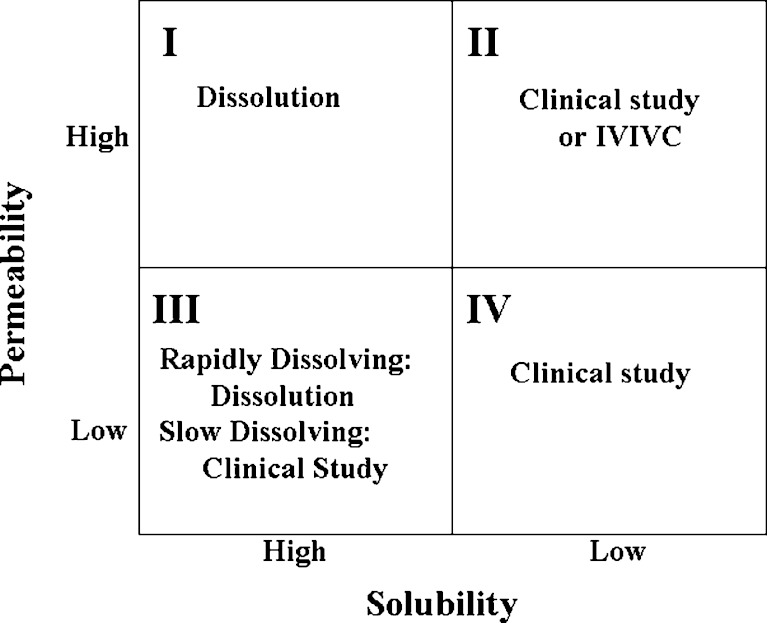
The use of the BCS to obtain a biowaiver of in vivo studies is often the first item that comes to mind when considering how the BCS is used in clinical development. However, the BCS also offers a framework from which to address the assessment of the adequacy of performance of new formulations through out clinical development. Such a strategy is illustrated in Fig. 1.
Fig. 1.
BCS framework for judging the adequacy of formulation performance
For BCS Class I compounds, dissolution may be used to judge the adequacy of a new formulation. Prior to pivotal efficacy and safety trials, there is no regulatory burden to establish bioequivalency. A company could chose to judge the adequacy of a new formulation using more limited dissolution testing, for example only comparing the new and old formulations at one pH between 1 and 7 where the compound is least soluble. When new formulations are introduced after pivotal efficacy and safety trials have begun, there is a need to establish bioequivalence. This can still be performed with dissolution; however such dissolution testing must conform to regulatory standards.
For BCS Class II compounds, dissolution is the rate limiting step to drug absorption and therefore dissolution can be used to judge the adequacy of performance with the caveat that the dissolution test used should reflect the in vivo performance. In other words it should be possible to develop an in vitro/in vivo correlation (IVIVC). Prior to pivotal efficacy and safety trials, it is possible to develop and use a correlation using a single formulation provided that there is sufficient confidence in the ability of the dissolution method to predict in vivo performance. This correlation should facilitate risk assessment and decision-making within pharmaceutical companies during formulation changes. Once pivotal efficacy and safety trials have started, assessment of new formulations would need to have an IVIVC that is validated to regulatory standards. Lack of an IVIVC would necessitate testing using clinical bioequivalence or bioavailability studies.
For Class III compounds that are rapidly dissolving one could use dissolution testing to judge adequacy of formulations introduced prior to pivotal efficacy and safety trials. For example, a new formulation may be considered acceptable if both the new and old formulations are more than 85% dissolved in 15 min at pH 1.2 as these conditions assure that the formulations dissolve rapidly in the stomach and that stomach emptying is the rate limiting step in the absorption process. For rapidly dissolving formulations introduced after pivotal efficacy and safety trials as well as non-rapidly (slowly) dissolving formulations, testing will need to conform to regulatory standards. It is worth noting that some agencies do allow dissolution based bioequivalence testing for Class III compounds (8). Finally for Class IV compounds, clinical bioequivalence and bioavailability studies will need to be performed.
Case Study II—A Case for Regulatory and Industrial Reform
Compound A is a development candidate currently being evaluated in late phase clinical studies. Its solubility is much greater than 1 mg/mL in aqueous medium in the pH range of 1.0 to 7.5. The amount of water needed to dissolve the highest dose strength is less than 2 mL. Based on the current guidance, Compound A is classified as a high solubility compound. Although the in vitro permeability (2.0 × 10−6 cm/s) based on the Caco-2 data was not considered high using metoprolol as the high permeability marker (16 × 10−6 cm/s), Compound A did yield an absolute oral bioavailability of more than 90% in human studies. Hence, Compound A is also classified as a high permeability compound. In addition, Compound A exhibits fast absorption with an early Tmax of <1 h in human with a solution formulation.
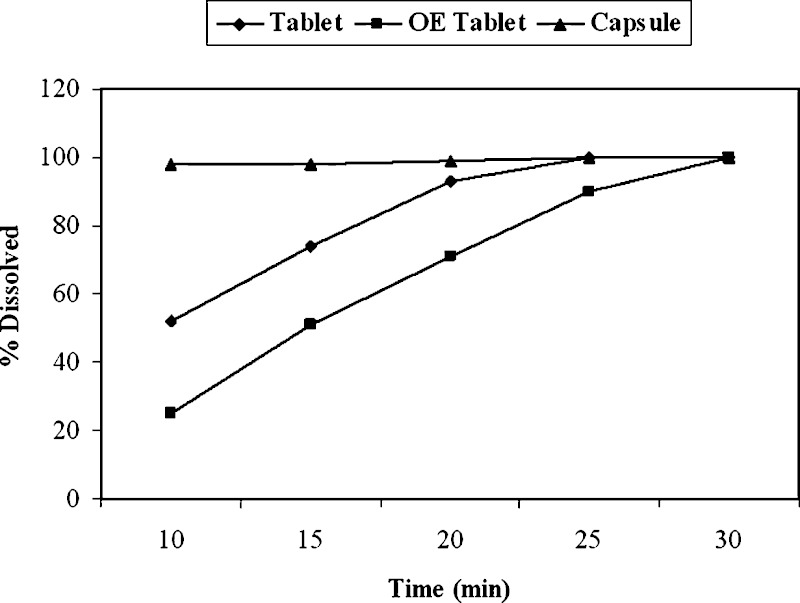
During the late phase development, the clinical formulation was changed from a tablet to a capsule formulation. Even though nearly complete release of Compound A was achieved at 30 min timepoint in 0.1 N HCl solution and in pH 4.5 and 6.8 buffers, noticeable differences in initial dissolution rate were observed among the capsule, tablet, and over-encapsulated (OE) tablet formulations with the latter being the slowest (Fig. 2). Specifically, both tablet and OE tablet formulation showed less than 85% drug release at 15 min. F2 values were less than 50 for various comparisons among the three formulations within the 30 min window. Among the three dose strengths, as expected, the dissolution rate difference was the greatest with the highest dose strength. Based on the current FDA guidance (9) for biowaiver using BCS, F2 values of >50 are required for biowaiver of in vivo studies if the drug release is less than 85% at 15 min at physiological pHs. Further, since BCS-based biowaiver approach is not employed to the same extent in EU and Japan in order to allow product filing around world simultaneously, biowaivers of in vivo studies for the three formulations were not pursued.
Fig. 2.
Differences in rate of dissolution among tablets, encapsulated tablets, and capsules of Compound A. The dissolution tests were carried out as described in the FDA guidance using USP Apparatus I at 100 rpm in three media (data in 10 mM phosphate buffer pH 6.8 shown above)
To assess the potential impact of very rapid dissolution of the new capsule formulation on pharmacokinetic (PK) profiles (especially the Cmax due to very early Tmax) of Compound A, a probe bioavailability (BA) study was conducted to compare the three formulations. The variabilities of pharmacokinetic parameters were assessed in this three-period crossover study with 18 healthy subjects. The dose tested was the highest clinical strength where the greatest differences in dissolution rate were observed. The geometric mean ratios (GMR) at 90% CI for AUC were very close to unity using the OE tablet as the reference formulation (which showed slowest initial dissolution). For the GMR for Cmax, the table formulation met the 0.8–1.25 target while the capsule formulation yielded a range of 0.96–1.27. A trend of higher Cmax value for the capsule formulation was detected in this study. Careful examination of the data showed that high Cmax value from a single subject received the capsule was the primary cause of the failed bioequivalence (BE). In addition, shorter Tmax values were detected for the tablet and the capsule formulation compared to the OE tablets. This probe study indeed confirm that for drugs with early Tmax the PK parameters are more sensitive to formulation changes and higher inter-subject variability can be expected.
Since OE tablet formulation had been used in pivotal efficacy studies, a subsequent definitive BE study for the OE tablet and capsule formulations was conducted. Given the observed difference and variability from the probe BA study, 90 subjects were employed in a two-period crossover design to have a 90% chance of declaring bioequivalence. In contrast to the results from the probe BA study, the two formulations were shown to be bioequivalent with geometric mean ratios for AUC and Cmax achieved nearly unity for this BCS class I compound. It should be noted that a trend of early Tmax for the capsule formulation was confirmed in the definitive BE study but this difference did not lead to bioinequivalence.
Since BE was achieved between the capsule and OE tablet at the highest dose strength despite the greatest difference in dissolution rate, it is expected that lower potency capsules should be bioequivalence to lower potency OE tablets. Based on the results from the definitive bioequivalence study conducted at the highest strength and the proportionally similar excipients used in all three potencies of the same type of formulation (10), waiver of additional BE studies for the two lower strengths was requested to FDA.
Discussion—A Need for Regulatory and Industrial Reform
As previously cited, approximately 30% of all oral immediate release drugs can be classified as highly permeable and highly soluble (11). Thus it may be surprising that between 2003 and 2006, only 25 requests (11 for NCEs and 14 for ANDAs) submitted to the FDA for either a BCS classification determination or for a waiver of an in vivo study (12). The perceived lack of certainty of acceptance by the regulatory agency has been cited as one reason for the reluctance to apply for biowaivers (13). This is in contrast with the traditional method of using in vivo studies to demonstrate bioequivalence. Pharmaceutical companies know how to run the studies and there is a historical record of regulatory acceptance that provides a sense of certainty. [It should be noted that only one of 11 applications for NCE for BCS classification was turned down by the US Food and Drug Administration (seven requests were granted; more information was requested by FDA for three other applications) (12).
As highlighted in the case of Compound A, there are regulatory barriers that prevent making biowaiver requests routine resulting in the conductance of expensive and time-consuming clinical BA/BE studies. The lack of uniformed employment of BCS-based biowaivers among US and EU/Japan agencies presents a major risk for WMA filing of a new product. Receiving a BCS-based biowaiver from the FDA due to formulation changes in late phase clinical studies may not assure a timely WMA filing. Even though agencies such as the FDA have made significant efforts in promoting the use of BCS-based biowaivers, the time required from filing a biowaiver request to receiving a formal approval from the FDA needs to be fast enough (e.g. within weeks rather than months) to the current uncertainty surrounding its approval when a pharmaceutical company decides to move a development program forward using a BCS-based waiver. Failure to receiving a biowaiver can lead to a significant delay in a development program. Hence, the risk associated with receiving a biowaiver approval often seems greater than benefit of saving resources.
A case may also be made that current guidances contain unnecessary barriers. The case study with Compound A clearly highlights the unnecessary requirement in the current BCS-based biowaiver guidance for F2 values of >50 if the formulations containing BCS class I drugs fail to release more than 85% of drugs in 15 min. The case with Compound A demonstrated that in the extreme scenario where larger differences in initial dissolution rate are detected and drugs have very early Tmax values BE are achieved for formulations that show >85% drug release in 30 min. However, given the pharmacokinetic characteristics of the compound, the differences in dissolution rates were not expected to result in bioinequivalent products (14). Hence, difference in dissolution profiles in the first 30 min may not be bio-relevant for rapidly dissolving formulations of BCS class I drugs.
Yet another barrier to biowaiver requests is the compartmentalization of company resources. The cost savings to an organization resulting from a biowaiver typically appears in the budget of the clinical department. However, departments such as preclinical pharmacokinetics, chemistry and formulation may be asked to perform more than the “normal” amount of work to support a biowaiver request. As a result there can be reluctance for all parts of the organization to support a biowaiver strategy. Further, for the compartmentalized large pharmaceutical companies, the reluctance for employing BCS-based biowaiver can also be caused by unclear responsibility for generating the biowaiver documents and accountability if a biowaiver request is rejected and program timeline is delayed.
For the reasons listed above, traditional BE approach of employing clinical studies is still often practiced as the preferred conservative option to ensure development timeline and to address regulatory uncertainty from various agencies around the world. Thus the full benefits of biowaivers to the pharmaceutical industry may not be fully achieved until pharmaceutical companies adopt models that better resource the support of biowaiver requests and regulatory agencies more fully align and create more timely processes.
CASE STUDIES III–V—APPLICATION OF BCS IN GENERIC DRUG DEVELOPMENT
Case Study III—Generic Drug Development of a Higher Strength Formulation
The first case study involved the request for the waiver of a bioequivalency study for a higher strength of a product for which a bioequivalency study had previously been submitted to the FDA. The reference product in this case was a tablet having three dosage strengths. The two lower strengths had previously been submitted to FDA based on a fasting and fed bioequivalency study utilizing the intermediate strength. Since literature suggested that the drug is highly soluble and highly permeable, a BCS Class I-based waiver of a fasting bio study for the high strength was desired. Confirmatory solubility studies showed that the drug is highly soluble per BCS criteria, and the reference product was shown to be rapidly dissolving. A rat perfusion study was then performed comparing test compound with a high permeability internal standard, and this study confirmed that the drug has high permeability. A stable dosage form having rapid dissolution was developed, and the ANDA for the higher strength was approved using the BCS approach.
Case Study IV—Generic Drug Development of a Generic Formulation Using Data Generated In-house
The second case study dealt with the submission of an ANDA containing a BCS based bioequivalency waiver request for a product for which the molecule was established as a BCS Class 1 compound through in-house experimentation. In this case the reference product was a tablet having one dosage strength, and the FDA recommendation was to conduct a fasting and food study to establish bioequivalence between test and reference products. Although the product labeling stated bioequivalence between an oral solution and the tablet, there was no reference in the label as to percent of oral dose absorbed. However, a literature search revealed that greater than 90% of an orally administered dose is absorbed. Solubility experiments showed the drug to be highly soluble per BCS criteria, and the reference product shown to be rapidly dissolving. Based on this information, a permeability study was performed via the Caco-2 model, and the compound was shown to be highly permeable by comparison to a highly permeable internal standard. As this information was being generated a stable and rapidly dissolving generic product was developed, and an ANDA was submitted with a request for a BSC biowaiver that was subsequently granted by the FDA.
Case Study V—Generic Drug Development of a Generic Formulation Using Literature Data
The final generic pharmaceutical case study involved the submission of an ANDA for a compound in which the literature clearly established the compound to be BCS Class 1. In this case, solubility and permeability work was performed to confirm literature findings in support of the ANDA submission. The reference product was a tablet with one dosage strength, and labeling for reference product stated that absolute oral bioavailability is approximately 100%. In addition, multiple literature references supported the fact that the drug is essentially completely absorbed after oral administration. Solubility experiments performed in-house showed the drug to be highly soluble per BCS criteria, and the reference product was shown to be rapidly dissolving. Based on this information it was decided to conduct a supportive permeability study, and the compound was shown to be highly permeable via the rat perfusion model by comparison to a highly permeable internal standard. A stable and rapidly dissolving generic product was developed, and an ANDA was submitted with a request for a BCS biowaiver which is currently under consideration by the FDA.
Discussion—Generic Drug Development
The establishment of the BCS and issuance of an FDA guidance document governing the use of the BCS to obtain waivers of bioequivalency studies for immediate-release solid oral dosage forms has made it possible for generic pharmaceutical companies to obtain FDA approval of some generic products without having to conduct a bioequivalency study comparing the generic and brand products. The advent of the BCS has thus made it possible for generic companies to perform drug development on certain products in a more time and cost-effective manner.
In order to execute a BCS development project, a generic company must first identify the target compound as a potential BCS Class 1 candidate through scientific information obtained during pre-formulation studies and from literature references. If the existing literature suggests that the compound is highly permeable, the solubility of the compound must then be confirmed through laboratory experiments. If the compound is found to be highly soluble per the FDA criteria for a highly soluble compound, then permeability studies are conducted to determine if the compound is highly permeable, typically through the use of an in situ animal model or the Caco-2 model. Based on these studies, if the compound is found to be highly soluble and highly permeable, the final objective for a BCS Class I ANDA submission is to develop a manufacturable, stable product that is rapidly dissolving.
It should be noted that the FDA Guidance for Industry allows for the use of a variety of methods to establish high permeability of a compound, including the use of the rat perfusion model as well as the Caco-2 model (9). It has been found that either method is suitable for use in establishing the permeability class of a compound; therefore both methods have been employed during the development of the products presented in Cases III–V. The use of a particular method was predicated upon a variety of factors, including prior literature information regarding the use of a particular method to determine permeability of the compound, development timelines, and availability of resources at outside contract research organizations to perform the studies.
The three case studies noted above demonstrate that the BCS can be strategically deployed to save time and resources during generic drug development. From a financial perspective, the avoidance of a bioequivalence study can be expected to save a generic company several hundred thousand dollars in development costs. In addition, use of the BCS can eliminate the need to expose human subjects to the test and reference products. From an overall timeline perspective there can be advantages to using the BCS approach assuming that required solubility, permeability and dissolution studies are performed in parallel with formulation development. If these studies are executed early in the development process, then several months of time can be shaved off of the overall development timeline. However, this approach should be selectively utilized, carefully considering regulatory risks versus benefit for each project considered for development. In particular, for compounds for which no published permeability literature exists, the risk of a potential rejection by the FDA of a submitted permeability study or a potential delay due to questions that may arise during review of the data must be weighed against the time and cost required to perform a human bioequivalency study. In these situations a case may be made that given the high probability of regulatory acceptance of a positive bioequivalence study, the use of a traditional approach is preferred in order to achieve a timely regulatory approval of the drug product.
CONCLUSIONS
The case studies presented clearly demonstrate that BCS-based biowaivers are becoming more routine and result in significant savings. The possibility also exists to expand application to realize even more savings. Yet just as clear as the benefits are the barriers that limit application: lack of international regulatory harmonization, uncertainty in regulatory approval, and organizational barriers within the pharmaceutical industry. Once these barriers are overcome and additional applications are fully allowed, the full benefits BCS applications will be realized.
References
- 1.Amidon G. L., Lennernäs H., Shah V. P., Crison J. R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Gupta E., Barends D. M., Yamashita E. Review of global regulations concerning biowaivers for immediate release solid oral dosage forms. Eur J Pharm Sci. 2006;29:315–324. doi: 10.1016/j.ejps.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Lyrica® (pregabalin capsules) [product information]. Pfizer, Inc, New York, NY, 2007, Jun.
- 4.U.S. Food and Drug Administration, December 30, 2004. NDA 021446. Lyrica (pregabalin) Capsules Clinical Pharmacology Biopharmaceutics Review(s). CDER, FDA.
- 5.European Medicines Agency, 2004. H-C-546. Lyrica (pregabalin) European Public Assessment Report, Scientific Discussion. EMEA.
- 6.Takagi T., Ramachandran C., Bermejo M., Yamashita S., Yu L. X., Amidon G. L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3:631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 7.Cook J. A., Bockbrader H. N. An industrial implementation of the biopharmaceutics classification system. Dissolution Technol. 2002;9:6–9. [Google Scholar]
- 8.D. M. Barends. BCS/BE in the Netherlands and Europe. Presented at the AAPS Workshop on BE, BCS, and Beyond, May 21– 23, 2007. North Bethesda, MD. Available at: http://www.aapspharmaceutica.com/meetings/files/90/35Barends.pdf. Accessed March 27, 2008.
- 9.Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. In: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (2000).
- 10.Guidance for Industry: Immediate release Solid Dosage Forms. Scale-Up and Postapproval Changes. In: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (1995).
- 11.Takagi T., Ramachandran C., Bermejo M., Yamashita S., Yu L. X., Amidon G. L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3:631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 12.M. Mehta. FDA Regulatory Use of BCS. Presented at the AAPS Workshop on BE, BCS, and Beyond, May 21–23, 2007. North Bethesda, MD. Available at: http://www.aapspharmaceutica.com/meetings/files/90/04Mehta.pdf. Accessed March 27, 2008.
- 13.Polli J. E., Yu L. X., Cook J. A. Summary workshop report: biopharmaceutics classification system—implementation challenges and extension opportunities. J Pharm Sci. 2004;93:1375–1381. doi: 10.1002/jps.20064. [DOI] [PubMed] [Google Scholar]
- 14.Yu L. X., Ellison C. D., Conner D. P., Lesko L. J., Hussain A. S. Influence of drug release properties of conventional solid dosage forms on the systemic exposure of highly soluble drugs. AAPS PharmSci. 2001;3(3):E24. doi: 10.1208/ps030324. [DOI] [PMC free article] [PubMed] [Google Scholar]