Table 1.
Exogenous, Catalytic Brønsted Base Additives for Direct Allylic C—H Amination
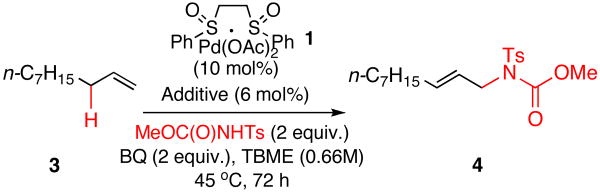 | ||||
|---|---|---|---|---|
| entry | additive | yield(%)a | L:Bb | E:Zc |
| 1 | none | 1d | - | - |
| 2 | (S,S)-Cr(salen)Cl 2 | 50 | 11:1 | 19:1 |
| 3 | pyridine | 9 | 12:1 | 14:1 |
| 4 | 2,6-di-tert-butylpyridine | - | - | - |
| 5 | N-isopropylamine | - | - | - |
| 6 | N,N-diisopropylamine | 66 | 12:1 | 15:1 |
| 7 | triethylamine | 60 | 14:1 | 15:1 |
| 8 | N,N-diisopropylethylamine | 66 | 11:1 | 17:1 |
| 9 | MeOC(O)NTsH-DIPEAe | 9 | 6:1 | 10:1 |
isolated yield of linear product.
linear/branched ratio determined by HPLC of crude reaction mixture.
determined by 1H NMR after chromatography.
1H NMR yield by comparison with internal standard.
2.0 equiv. of the pre-formed salt was used instead of MeOC(O)NHTs.
