Table 2.
Optimization of Reaction Conditions for the Preparation of Potassium 4-Azidophenyltrifluoroborate (1b)a
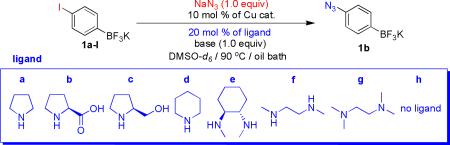 | |||||
|---|---|---|---|---|---|
| entry | CuX | ligand | base | reaction time (h) | conversion yield (%)b |
| 1 | CuI | a | K2CO3 | 24 | 66 |
| 2 | CuI | b | K2CO3 | 5 | 98 |
| 3 | CuI | c | K2CO3 | 16 | 100 |
| 4 | CuI | d | K2CO3 | 24 | 81 |
| 5 | CuI | e | K2CO3 | 15 | 100 |
| 6 | CuI | f | K2CO3 | 1 | 100 (88)c |
| 7 | CuI | g | K2CO3 | 24 | trace |
| 8 | CuI | h | K2CO3 | 48 | 68 |
| 9 | CuId | f | K2CO3 | 2 | 100 (89)c |
| 10 | CuBr | f | K2CO3 | 1 | 100 (92)c |
| 11 | CuBr | f | Cs2CO3 | 0.5 | 100 (94)c |
| 12e | CuBr | f | Cs2CO3 | 2.5 | 100 |
All reactions were performed on a 0.05 mmol scale in 0.5 mL of DMSO-d6 in an NMR tube.
Percent conversions were determined by 1H NMR of the reaction mixtures. The conversion yield was based on the integration of peaks at 7.14 (1a-I) ppm and 6.85 (1b) ppm, respectively.
Reactions were performed on a 0.1 mmol scale and isolated yields are reported.
5 mol % of CuI was used.
Reaction was performed in DMF-d7.
