Table 4.
Cross-Coupling Reactionsa
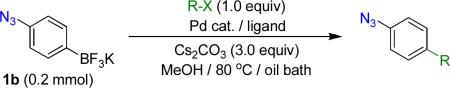 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R-X | reaction conditions | reaction time (h) | product | yield | ||
| 1 | 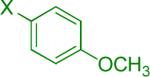 |
A B |
 |
0.5 6 |
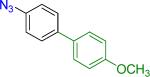 |
17 | 85% – |
| 2 | 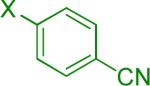 |
A B |
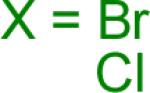 |
1 6 |
 |
18 | 83% 48% |
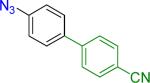
| 3 | 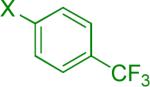 |
A B |
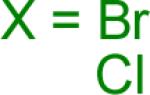 |
0.5 3 |
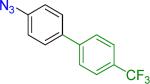 |
19 | 58% 45% |
| 4 | 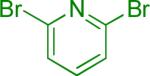 |
A | 3 | 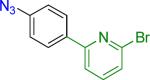 |
20 | 81% | |
Reaction conditions A: potassium 4-azidophenyltrifluoroborate (1b, 0.2 mmol), aryl bromide (0.2 mmol), 10 mol % of PdCl2(dppf)·CH2Cl2, Cs2CO3 (0.6 mmol), MeOH (1.5 mL), 80 °C.; B: 1b (0.2 mmol), aryl chloride (0.2 mmol), 3 mol % of Pd(OAc)2, 6 mol % of XPhos, Cs2CO3(0.6 mmol), 1,4-dioxane/H2O (10/1, 1.5 mL), 100 °C. Yields are given for isolated products.
