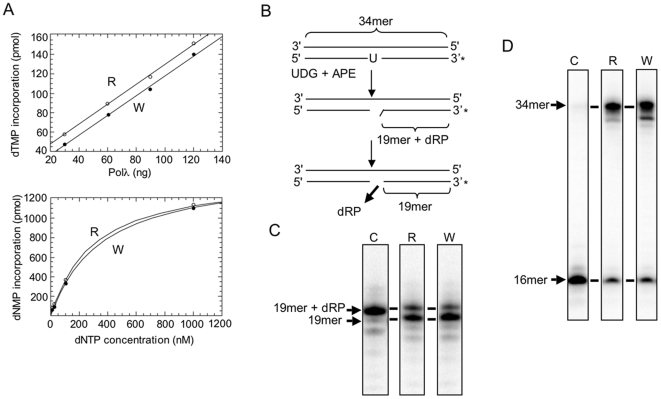
Figure 2. Enzymatic activities of the hPolλ W438 variant.
(A) DNA polymerization activity of the two allelic variants of hPolλ. DNA polymerase activity of each independent hPolλ variant (R438 or W438), measured as dNMP incorporation on activated DNA, was estimated as a function of enzyme (top) and dNTP (bottom) concentration, as described in Materials and Methods. The first experiment, in which the only dNTP provided was TTP (13,3 nM), demonstrates that nucleotide insertion was linear for both variants, in the interval of 30–120 ng of enzyme. The second experiment, using 60 ng of each enzyme variant, tested polymerization of the four dNTPs at various (from limiting to saturating) concentrations. (B–D) dRP lyase activity and reconstitution of BER in vitro with the two allelic variants of hPolλ: (B) The scheme shows a 34-mer double-stranded oligonucleotide containing an uracil residue (at position 16) in the strand which is 3′-end labeled (*). After treatment with UDG and hAPE, a dRPcontaining nicked substrate (19mer+dRP) is obtained, that can be a substrate for dRP lyase activity. (C) In vitro analysis of the dRP lyase reaction. As shown in the autoradiogram, the dRP moiety can be cleaved by incubation with either variant (R or W) of hPolλ (30 nM). (D) In vitro reconstitution of a BER reaction. A non-labeled 34-mer double-stranded oligonucleotide containing a uracil residue at position 16 in one strand is treated with UDG (100 nM) and hAPE (40 nM) to release a dRP-containing nicked substrate. By adding a labeled dNTP (α-dCTP) and either purified hPolλ R or hPolλ W variants (60 nM), two labeled products can be observed after denaturing electrophoresis and autoradiography: (i) a 16-mer product generated by a single nucleotide insertion at the 3-hydroxyl end of the 5-incised AP site; (ii) a 34-mer product that corresponds to the complete repair of the DNA strand upon T4 DNA ligase action.