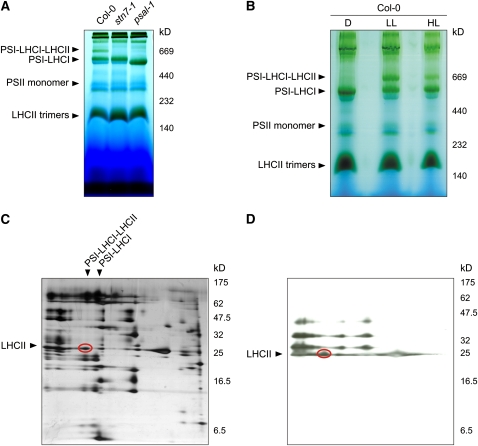
Figure 2.
Visualization of the PSI-LHCI-LHCII Complex Associated with State 2–Adapted Thylakoid Membranes.
(A) BN-PAGE of identical amounts of thylakoid proteins isolated from wild-type (Col-0), stn7-1, and psal-1 leaves adapted to low-light conditions (80 μmol m−2 s−1 for 8 h). A pigment-protein complex (PSI-LHCI-LHCII) migrating at ∼670 kD is clearly visible in wild-type (Col-0) thylakoids, whereas it is completely absent in stn7-1 and psal-1 thylakoids; both mutants are blocked in state 1. Note that the PSI-LHCI protein complex from psal-1 thylakoids appears to be smaller than in wild-type (Col-0) and stn7-1 plants, as a consequence of the altered polypeptide composition of PSI (see also Figure 10).
(B) BN-PAGE (as in [A]) of thylakoid proteins isolated from wild-type (Col-0) leaves adapted to darkness for 16 h (D), low light (LL; 80 μmol m−2 s−1 for 3 h), or high light (HL; 800 μmol m−2 s−1 for 3 h).
(C) The wild-type low-light lane from a BN gel was subjected to denaturing PAGE, and the 2D gel was stained with silver. The polypeptide fractionation shows that the ∼670-kD pigment-protein complex consists of PSI and LHCI subunits, together with a portion of LHCII polypeptides (oval).
(D) Thylakoid protein phosphorylation detected by immunoblot analysis with a phosphothreonine-specific antibody. Thylakoid membranes isolated from low light–adapted wild-type (Col-0) leaves were fractionated (as in [C]). The LHCII polypeptides associated with PSI-LHCI complexes are highly phosphorylated (oval).