Figure 1.
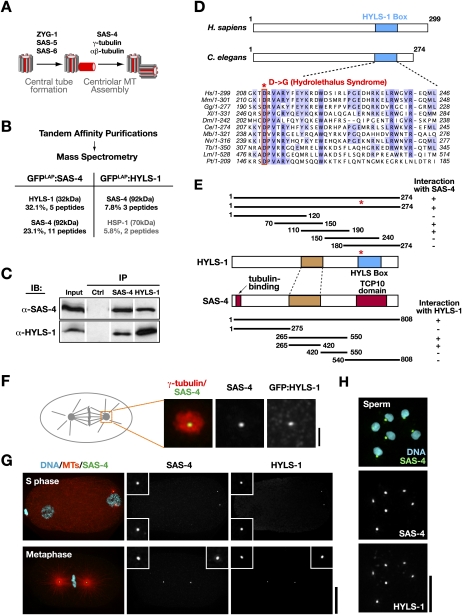
HYLS-1 directly associates and colocalizes with the core centriolar structural protein SAS-4. (A) Schematic of centriole assembly pathway in C. elegans: The central tube, a cylindrical intermediate that templates centriole dimensions, assembles in S phase via an SAS-4-independent process. SAS-4 is recruited to the central tube and directs the assembly of the stabilized microtubules that constitute the outer centriole wall during mitotic prophase (Pelletier et al. 2006; Dammermann et al. 2008). (B) Results of mass spectrometry performed on tandem affinity preparations of SAS-4 and HYLS, listing all polypeptides above 5% coverage. (C) Immunoblot showing SAS-4 and HYLS-1 coimmunoprecipitate from embryo extracts. (D) Schematics of human and C. elegans HYLS-1, indicating position of conserved “HYLS-1 box.” Alignment shows HYLS-1 orthologs from vertebrates, Drosophila, C. elegans, and ciliates highlighting invariant aspartic acid mutated in hydrolethalus syndrome. (E) SAS-4 and HYLS-1 directly interact in a yeast two-hybrid assay via their middle portions (brown). For reference, the location of the conserved HYLS-1 box (blue), and the N-terminal tubulin-binding and C-terminal TCP10 motifs in SAS-4 (red), are also shown. The hydrolethalus-associated mutation does not affect interaction with SAS-4. (F) Centrosome from a metaphase embryo stained with antibodies to γ-tubulin as a marker for the pericentriolar material, SAS-4 and GFP. GFP:HYLS-1 colocalizes with SAS-4 at centrioles, which appear as a small focus in the center of the pericentriolar material. (G,H) HYLS-1 colocalizes with SAS-4 to centrioles throughout the embryonic cell cycle (G) and in sperm (H). Bars: F, 1 μm; G, 10 μm; H, 5 μm. Insets in G are magnified 3×.