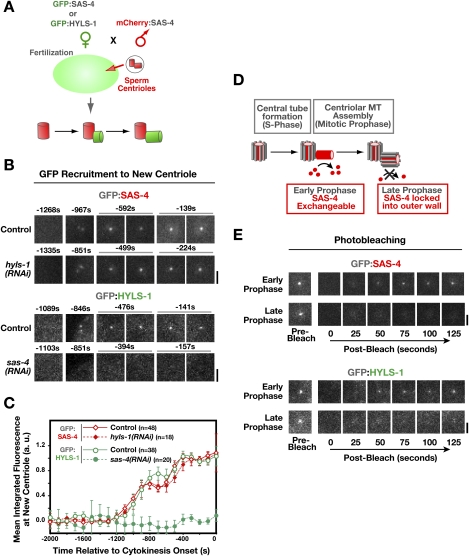
Figure 2.
HYLS-1 is stably incorporated into newly forming centrioles in an SAS-4-dependent manner. (A) Schematic of the method used to analyze recruitment of centriolar proteins to site of centriole assembly (Dammermann et al. 2008). Briefly, sperm centrioles labeled with mCherry:SAS-4 were introduced by mating into oocytes expressing a GFP fusion with HYLS-1 or SAS-4. Recruitment of the GFP-labeled protein to newly forming centrioles was quantified by measuring the GFP fluorescence coincident with the mCherry signal at each time point. Measurements from multiple embryos were pooled and averaged to generate each recruitment plot. (B,C) Representative images (B) and quantification (C) of centriolar GFP:SAS-4 or GFP:HYLS-1 recruitment in control embryos and embryos depleted of HYLS-1 or SAS-4 as indicated. HYLS-1 recruitment requires SAS-4 but not vice versa. Times are in seconds relative to cytokinesis onset. Error bars are the 90% confidence interval. (D) SAS-4 is recruited to centrioles during the formation of the central tube in S phase, but remains in dynamic exchange with the cytoplasmic pool of SAS-4 until assembly of the centriolar microtubules in late prophase locks it into the outer centriole wall (Dammermann et al. 2008). (E) GFP:SAS-4 and GFP:HYLS-1 exhibit an identical behavior in a photobleaching-based assay assessing the ability of the centriolar protein to exchange with cytoplasmic pools. Recovery is observed if centrioles are bleached in early prophase (GFP:SAS-4, n = 8 out of 8; GFP:HYLS-1, n = 8 out of 8), but not if centrioles are bleached in late prophase (GFP:SAS-4, n = 1 out of 22; GFP:HYLS-1, n = 0 out of 9). Bars, 2 μm.