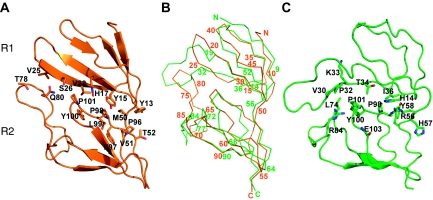
Figure 2.
R1–R2 interdomain interactions. (A) R1–R2 contacts found in the R1–R7 structure determined in this work. The contacting residues at the interface are shown as sticks and labelled. Strands β2 and β3 of domain R1 form polar and hydrophobic interactions with the R2 residues located in the β2–β3 loop, strand β4 and in the interdomain linker. (B) View of the α-carbon trace of the recombinant R1–R7 (orange) with every five residues numbered. The structure of the equivalent region in the 3.5-Å structure of the complete vault (Tanaka et al, 2009) is superimposed (green). The residue numbers are written when structural correspondence was observed. (C) R1–R2 contacts found in the 3.5-Å structure.