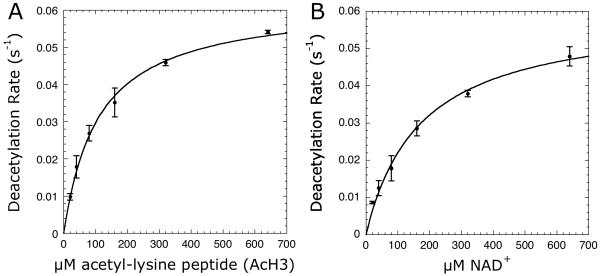
Figure 3.
Steady-state kinetics of Sirt1 varying (A) AcH3 or (B) NAD+ concentrations. Reaction mixtures either contained 20-640 μM AcH3 with 640 μM NAD+ or 20-640 μM NAD+ with 640 μM AcH3 in 0.2 mM NAD(P)H, 1 mM DTT, 3.3 mM α-ketoglutarate, 2 μM MBP-PncA, ∼2 units of glutamate dehydrogenase from Proteus, 0.5 μM Sirt1 in 20 mM potassium phosphate at pH 7.5. Background rates measured in absence of the varied substrate were subtracted from the Sirt1 catalyzed reactions. To determine kcat and Km values, the plot of steady-state nicotinamide formation rates versus substrate concentration was fit to the Michaelis-Menten equation [v = (kcat · [S])/(Km + [S])] using KaleidaGraph (Synergy Software, Reading, PA).