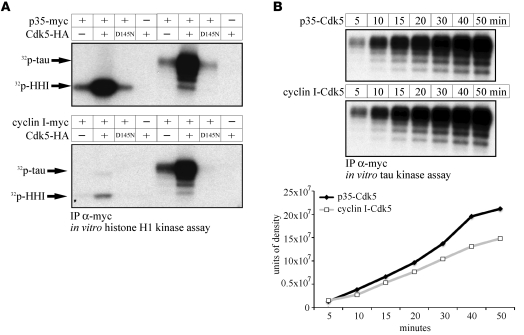
Figure 3. Cyclin I–Cdk5 preferentially phosphorylates tau.
(A) HEK293 cells were transfected with HA-Cdk5, then cotransfected with either myc-p35 or myc–cyclin I. Following an IP to the myc epitope tag on either p35 or cyclin I, in vitro kinase assays were performed using either histone H1 (HH1) or tau as substrates. Histone H1 and tau (lanes 2, 6) were strongly phosphorylated by p35-Cdk5. In contrast, cyclin I–Cdk5 preferentially phosphorylated tau (lane 6) rather than histone H1 (lane 2). No kinase activity was detected in the presence of a kinase-inactive dominant negative Cdk5 mutant (HA-D145N-Cdk5, lane 3, 7) or in the absence of either Cdk5 (lanes 1, 5) or p35/cyclin I (lanes 4, 8). (B) To compare the kinase kinetics by which cyclin I–Cdk5 phosphorylates tau in comparison with p35-Cdk5, a time-course experiment was performed in HEK293 cells cotransfected with myc-p35 and HA-Cdk5 or myc–cyclin I and HA-Cdk5. Following IP for the myc tag, phosphorylation of tau was assessed by incorporation of 32P-ATP quantified on a phosphoimager system. Cyclin I–Cdk5 showed phosphorylation kinetics similar to those of p35-Cdk5. Phosphorylation of tau was already evident after 5 minutes and reached a plateau after 40–50 minutes.