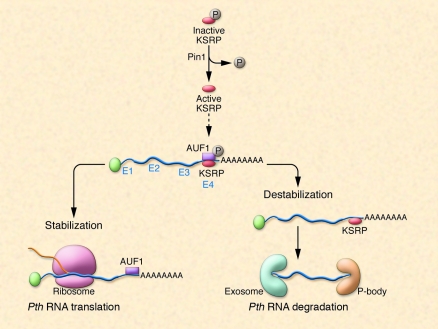
Figure 2. Processing of Pth mRNA.
Murine Pth mRNA is bound by ARE-BPs, which either stabilize or destabilize Pth mRNA, thereby altering Pth mRNA half-life. The ratio of activities of stabilizing/destabilizing ARE-BPs bound to Pth mRNA determines the half-life of a given Pth mRNA molecule. KSRP is a Pth mRNA–destabilizing ARE-BP that is active in its dephosphorylated state. In their new study in this issue of the JCI, Nechama et al. (46) report that the peptidyl-prolyl isomerase Pin1 is responsible for the dephosphorylation of KSRP. In CKD, Pin1 activity is reduced, and as a result, less dephosphorylated (active) KSRP is available. As a consequence, a stabilizing ARE-BP, AUF1, is active and Pth mRNA is degraded to a lesser extent, resulting in higher intracellular Pth mRNA levels, more PTH synthesis, and secondary hyperparathyroidism. P, phosphate.