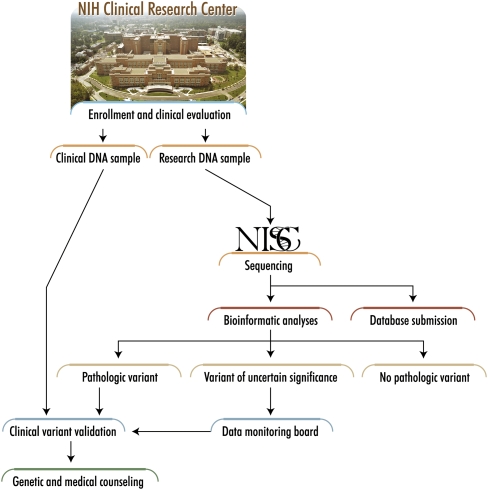
Figure 2.
ClinSeq sample and data flow. DNA samples and clinical data emanate from the initial participant enrollment and clinical evaluation, and then flow through the indicated clinical and research processes (see text for details). Note the separate acquisition and handling of DNA samples for clinical and research purposes, respectively, with the former handled by a CLIA laboratory prior to any results being returned to participants. Further, variants identified in putative disease-causing genes must first be reviewed by a data-monitoring board before being reported back to participants.