Figure 6. Role of EPRS Linker Phosphorylation in Binding the GAIT RNA Element and GAIT-mediated Translational Silencing.
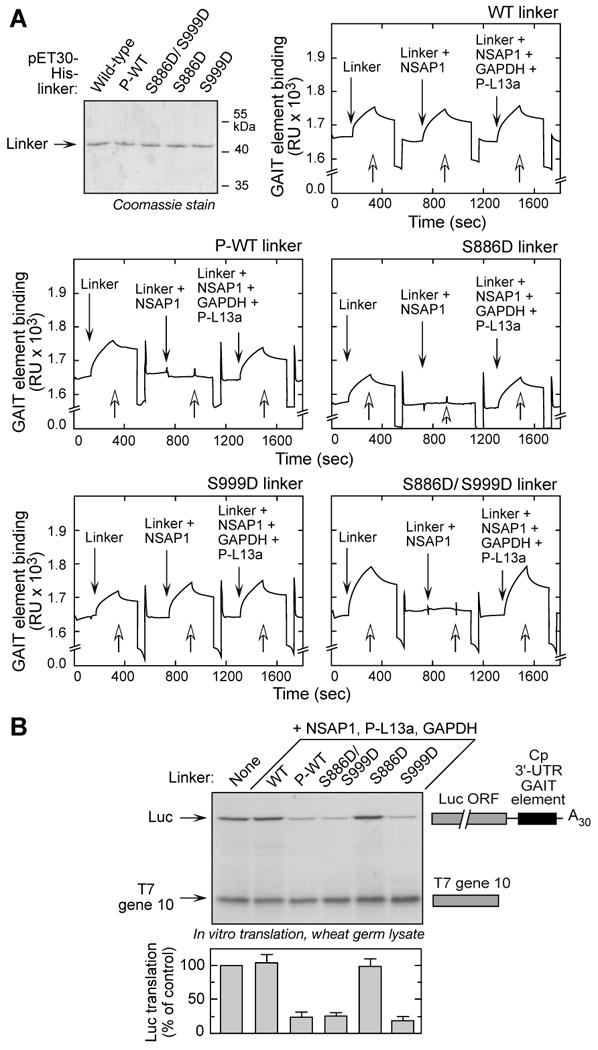
(A) Ser886 phosphorylation is sufficient for reconstituting binding to GAIT RNA element. N-terminus, His-tagged wild-type (WT) and phospho-mimetic (Ser-to-Asp, S-D) mutant linkers were expressed in E. coli and purified by Ni-affinity chromatography. Phosphorylated, wild-type (P-WT) linker was obtained by in vitro phosphorylation of purified protein with lysates of 4-h, IFN-γ-treated U937 cells, and repurification by Ni-affinity column. Biotinylated Cp GAIT RNA element was immobilized on a streptavidin sensor chip, and binding of linker to RNA was determined by SPR and expressed as resonance units (RU). After removal of bound protein with wash buffer (open arrows), binding of linkers preincubated with NSAP1 was measured, then linkers preincubated with NSAP1, GAPDH, and phospho-L13a (P-L13a) (each at 10-fold molar excess).
(B) Ser999 phosphorylation is critical for GAIT complex activation. His-tagged, wild-type (WT), phosphorylated-WT (P-WT), and phospho-mimetic linker proteins were preincubated with His-tagged NSAP1, GAPDH, and phosphorylated, His-tagged L13a (P-L13a), and then added to wheat germ extract for in vitro translation of luciferase (Luc)-Cp-GAIT element reporter in presence of 35S-labeled Met. T7 gene 10 RNA was co-translated as a specificity control. Luc expression was quantified by densitometry, normalized by T7 gene 10 translation, and expressed as % of control (mean ± SEM, n = 3 experiments).
