Abstract
While it has long been known that human CD4+ T cells can express functional class II MHC molecules, the role of lysosomal proteases in T cell class II MHC processing and presentation pathway is unknown. Using CD4+ T cell clones that constitutively express class II MHC, we determined that cathepsin S is necessary for invariant chain proteolysis in T cells. CD4+HLA-DR+ T cells downregulated cathepsin S expression and activity 18 hours after activation, thereby ceasing nascent class II MHC product formation. This blockade resulted in the loss of the invariant chain fragment CLIP from the cell surface, suggesting that—like professional APC—CD4+ HLA-DR+ cells modulate self-antigen presentation as a consequence of activation. Furthermore, cathepsin S expression and activity, and concordantly cell surface CLIP expression, was reduced in HLA-DR+ CD4+ T cells as compared to B cells both in vitro and ex vivo.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Introduction
CD4+ T cells are activated by T cell receptor engagement of peptide/class II MHC complexes on antigen presenting cells (APC) to initiate an adaptive immune response, but can themselves also express class II MHC (1, 2). The expression of class II MHC on CD4+ T cells occurs in most mammalian species(3), the exception being mice, which do not transcribe the class II transactivator (CIITA) promoter III in CD4+ T cells(4, 5).
In the human system, expression of HLA-DR—the most prevalent Class II MHC molecule—was first described as a marker of activated T cells(2). Patients with chronic autoimmune disease, inflammation, and the recent recipients of immunizations exhibited a higher frequency of HLA-DR+ T cells in the peripheral blood as compared to healthy donors(6). Yet for human CD4+ T cells, HLA-DR is more than a biomarker of activation. Class II MHC on these cells is functional and can be used to present peptide antigen to activate responder CD4+ T cells in vitro (7–9). Furthermore, recent studies have identified HLA-DR expression on CD4+ T cells in the blood of healthy donors, specifically a subset of CD4+ CD25hi FoxP3+ natural regulatory T cells, and suggest that HLA-DR may have a functional role in these cells (10).
Although the class II MHC processing and presentation pathway has been studied extensively in professional APC, this pathway in human CD4+ T cells has not been characterized. This is not a trivial issue, as many of the enzymes involved in the generation of antigenic peptides are not ubiquitously expressed. HLA-DR maturation is regulated by the invariant chain (Ii), which acts as a surrogate substrate and trafficking chaperone(11). As the MHC:Ii complex migrates through the endo/lysosomal compartment, resident proteases systematically degrade the this chaperone, leaving only the Ii fragment CLIP to occlude the class II MHC binding pocket. These same proteases hydrolyze self and foreign proteins to generate peptide epitopes, which ultimately displace CLIP and are loaded into the class II MHC binding pocket with the aid of the loading molecule HLA-DM(12).
Key proteolytic regulators of Class II MHC processing have been identified in professional APC with the use of knock-out mice and specific protease inhibitors(13, 14). Blockade of Ii degradation results in the accumulation of Ii intermediates and can lead to a corresponding decrease in surface expression of class II MHC products(15, 16). In human B cell lines, treatment with the pan-cysteine protease inhibitor leupeptin or the cathepsin S inhibitor leucine-homophenylalanine-vinyl sulfone (LHVS) blocks successful degradation of Ii (17). Characterization of CatS knock-out mice has further implicated CatS in the terminal cleavage of Ii to yield CLIP in professional APC (13). Further studies, however, have demonstrated a cell-type specific role for cysteine proteases in these later stages of Ii processing. Cathepsin L (CatL) in thymic epithelial cells and cathepsin F (CatF) in macrophages can also perform this cleavage(18) (19).
Although B and T cells are derived from a common precursor, these cells ultimately differentiate into functionally unique lineages with distinct trafficking pathways, organization, and composition within their intracellular processing compartments. This prompted us to explore in detail the biosynthesis of human class II MHC products in MHC-identical B and T cells. Here we demonstrate, using CD4+ T cell clones, that CatS is a key enzyme required for proteolysis of Ii in CD4+ HLA-DR+ T cells. We find that activation-induced regulation of CatS expression and activity leads to the downregulation of CLIP expression in CD4+ HLA-DR+ T cells both in vitro and ex vivo. Our data indicates that CD4+ HLA-DR+ T cells modulate peptide epitope presentation post-activation, and furthermore suggests that presentation of non-CLIP self-peptide may be integral to the function of class II MHC on these cells.
Materials and Methods
Cell culture reagents and antibodies
Cells were cultured in RPMI 1640 medium supplemented with 2mM L-glutamine, 5 mM HEPES, 100 U/ml penicillin/streptomycin (all from BioWhittaker), 0.5 mM sodium pyruvate, 0.5mM non-essential amino acids (from Gibco) in 96-well U-bottom plates or 25cm2 vented flasks (CoStar). T cell clone media additionally received 5% human AB serum (Cellgro Meditech Inc., Hendon, VA) and 25 U/ml recombinant human IL-2 (Tecin, NCI). The media for the EBV-transformed B cell lines was supplemented with 8% fetal bovine serum. The αCD3 (UCHT1 and Hit3a), αCD4 (RPA-T4), αCD28 (CD28.2 and 3D10), αCLIP/HLA-DR (CerCLIP), αHLA-DR (L243 and Tü36), αClass II MHC (Tü39), αHLA-DM (MaP.DM1), and αCD19 (1D3) antibodies were purchased from BD Pharmingen.
Cell isolation
Whole mononuclear cells were isolated from healthy individuals after informed consent in green-capped, heparinized tubes by Ficoll-Hypaque (GE Healthcare Bio-Sciences, UK) gradient centrifugation. CD19+ B cells were isolated using CD19 Microbeads (Miltenyi Biotec). Total CD4+ T cells were isolated via the CD4+ T cell negative isolation kit II (Miltenyi Biotec) and incubated with an excess volume of fluorochrome-labeled antibodies against HLA-DR (L243 PerCP), CD62L (Dreg 56 APC), CD25 (M-A251 PE), CD32 (3D3 FITC), CD14 (M5E2 FITC), CD116 (M5D12 FITC), and CD20 (2H7 FITC) all from BD Pharmingen. The FITC-labeled antibodies were used as a combined cocktail to ensure that no antigen presenting cells were isolated. HLA-DR+ CD4+ T cell populations were sorted on a FACS ARIA (BD Biosciences) to typically >98% purity in post sort analysis.
CD4+ T cell clones and Generation of EBV transformed B cell lines
T cell clones were generated from the peripheral blood of healthy individuals [DRB1*01,03, DRB3*; DRB1*15,13, DRB3*, DRB5*; DRB1*15,07, DRB4*, DRB5*]. CD62L+CD25−HLA-DR−CD4+ cells were sorted at one cell per well into 96-well U bottom plates containing 2×105 irridiated (5000 rad) PBMC, 50 U/ml recombinant human IL-2, 1 μg/ml αCD3 (Hit3a), and 1 μg/ml αCD28, a modification to previously published procedures(10). Clones were expanded for 30 days before restimulation in 96-well U bottom plates coated with 50μl of 1 μg/ml each αCD3 (UCHT1) and αCD28 (3D10) diluted in PBS, incubated for 2 hours at 37°C, and then washed once in PBS. Clones were expanded for 4 weeks in XVIVO-15 medium and then subsequently restimulated in 25cm2 flasks coated with αCD3 and αCD28. Clones underwent at least two more rounds of expansion and restimulation with αCD3 and αCD28 prior to assay. EBV-transformed B cell lines were generated from peripheral blood mononuclear cells as previously described(20).
Gene expression analysis
RNA was isolated by the RNeasy Mini Kit, RNAse-free DNAse procedure (Qiagen), converted to cDNA via reverse transcription by random hexamers and Multiscribe RT using the TaqMan RT-PCR kit from Applied Biosystems Inc. and diluted 1/10 before use. TaqMan PCR reactions were performed in triplicate using TaqMan Fast Universal PCR Master Mix to amplify human CIITA (Hs00172106_m1), HLA-DRα (Hs00219575_m1), CatB (Hs00157194_m1), CatL (Hs00377632_m1), CatS (Hs00356423_m1), and CatV (Hs00426731_m1), GADPH (Hs99999905_m1), or β2M (4326319E) on an ABI 7500 instrument (all from Applied Biosystems). Difference in Ct values normalized to β2M for each sample as per the formula: Normalized expression = 0.5^((−Ct value of β2M – Ct value of target )*1000).
Pulse-chase and immunoprecipitation
Radiolabeling experiments were conducted as previously described(21). Briefly, cells were washed and incubated in methionine-free medium (Gibco) for 1 hour at 37°C. During the last 10 minutes of starvation, cells were treated with control amounts of DMSO, 1 mM leupeptin (all from Sigma-Aldrich), or LHVS as indicated. These concentrations of inhibitor were maintained throughout the remainder of the pulse and chase. Following starvation, cells were pulsed with 0.5 mCi of [35S]methionine (PerkinElmer) for 45 minutes and then chased with regular culture medium for the time indicated. After the chase, cells were washed once with PBS and lysed. Precleared lysates were incubated with Tü36 and protein A-agarose beads (Roche) to immunoprecipitate class II MHC molecules. Samples were boiled in sample buffer prior to analysis on a 12.5% SDS-PAGE gel.
Substrate-specific protease activity assays and active-site labeling
The enzyme activity of cathepsin B, cathepsin B plus L, cathepsin S, and AEP was measured in vitro as described(21, 22). DCG-04 labeling was performed as described(21, 23).
Results
Establishment of CD4+ HLA-DR+ T cell clones
Class II MHC is a traditional biomarker of activated human CD4+ T cells(2), but relatively little is known about endogenous class II expression, processing and antigen presentation in these adaptive, non-professional APC. In order to assess class II MHC expression in CD4+ T cells at the single cell level, we generated CD4+ T cell clones from the peripheral blood of healthy donors. We propagated these clones in APC-free cultures to ensure that our analysis was restricted to endogenous class II MHC expression and would exclude acquisition of class II from traditional APC. We compared class II MHC synthesis in these clones to genetically identical EBV-transformed B cell lines. This comparison allowed us to control for donor-to-donor variability in protein expression, enzyme activity, and MHC haplotype.
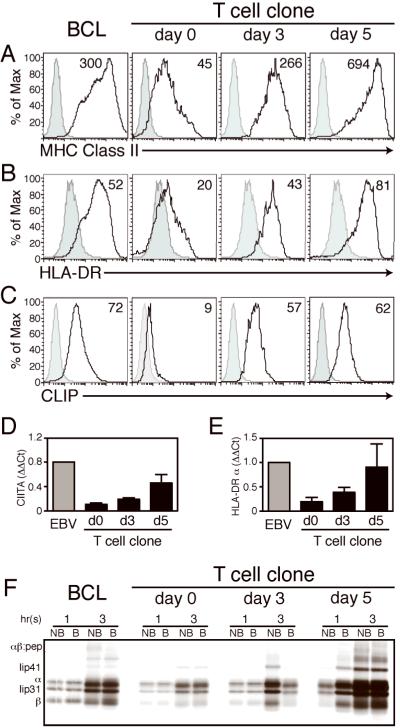
Consistent with previous reports(24, 25), chronically activated CD4+ T cell clones acquired constitutive cell surface expression of the class II MHC determinants HLA-DR, HLA-DP, and HLA-DQ (Fig 1A–B, data not shown). This basal level of class II expression was upregulated by polyclonal activation of T cell clones with αCD3 and αCD28 (Fig 1). After 5 days of activation, these clones expressed cell surface class II MHC equivalent to that of EBV-transformed B cells. Additionally, activated CD4+ T cell clones upregulated class II transactivator (CIITA) and HLA-DRα mRNA after treatment with αCD3 and αCD28 (Fig 1D–E), in tandem with cell surface protein expression. We confirmed that expression of Class II MHC was endogenous by metabolic labeling with [35S]-methionine, followed by immunoprecipitation of HLA-DR in resting and activated T cell clones (Fig 1F). Synthesis of HLA-DRα and β chains, as well as Ii isoforms p41 and p31, was upregulated at 3 and 5 days post-activation in these cells, although SDS-stable dimer formation was not observed for all individuals assayed. These results indicate that human CD4+ T cell clones synthesize and express class II MHC, corroborating previous reports of HLA-DR expression by CD4+ T cell clones (7, 9).
Figure 1. The constitutive expression of class II MHC in CD4+ T cell clones is upregulated following activation.
Donor-matched BCL and a representative CD4+ T cell clone were activated with cross-linking αCD3/αCD28 and assayed for cell surface expression of (A) total class II MHC, (B) HLA-DR, and (C) HLA-DR:CLIP complexes. Isotype control shown in grey. Also shown, quantification of relative mRNA expression in BCL and CD4+ T cell clones by Taqman RT-PCR of (D) the class II transcriptional activator and (E) HLA-DRα chain in BCL and T cell clones. (F) Endogenous synthesis of HLA-DRα, HLA-DRα, and Ii was confirmed by metabolic [35S]-methionine labeling, followed by immunoprecipitation of HLA-DR complexes with the conformationally specific antibody Tü36, and SDS-PAGE under the conditions indicated (B, denatured by boiling; NB, non-boiled). Histogram and mean fluorescence intensity (MFI) of representative samples shown for A–D; for E–F, graphs represent mean±SEM, n = 4 donors.
Class II MHC processing in CD4+ T cell clones requires cysteine proteases
We detected the Ii fragment CLIP on the surface of CD4+ T cell clones (Fig. 1C) and Ii isoforms bound to HLA-DR complexes immunoprecipitated from [35S]-methionine labeled cells (Fig 1F). Given these findings, we hypothesized that CD4+ T cell clones, like professional APC, utilize the endosomal Class II MHC processing pathway. In this pathway, HLA-DR α and β chains are assembled on the Ii chaperone, which must be processively cleaved by resident proteases in the endo-lysosomal compartment to allow peptide loading.
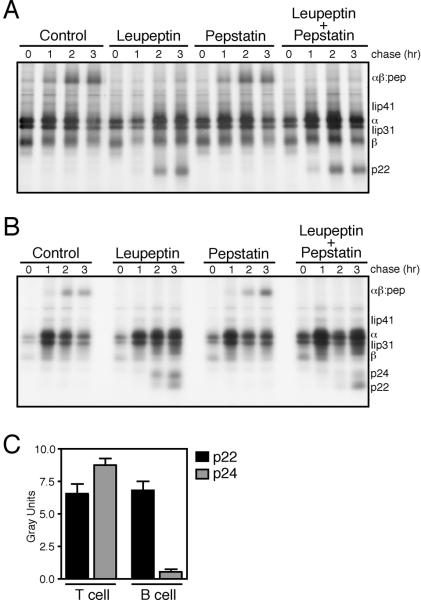
To identify a proteolytic requirement for Class II MHC αβ dimer formation in CD4+ T cell clones, we treated these cells with the pan-cysteine protease inhibitor leupeptin or the pan-aspartyl protease inhibitor pepstatin, pulsed the treated cells for 45 minutes with [35S]-methionine to label nascent proteins, and then chased the radiolabeled proteins for up to 3 hours before immunoprecipitation of HLA-DR complexes and associated Ii fragments. In EBV-transformed B cells, treatment with leupeptin but not pepstatin inhibited Ii cleavage; blockade of cysteine proteases in these cells generated a 22kDa peptide (the p22 leupeptin induced peptide, LIP) and prevented successful SDS-stable dimer formation (αβ:peptide) (16) (Fig. 2A). Likewise, leupeptin treatment, but not pepstatin treatment, inhibited HLA-DR maturation in a donor-matched CD4+ T cell clone (Fig. 2B). After two hours of chase, the Ii fragments p22 and p24 could be resolved in T cell clones treated with leupeptin alone but not in those treated with pepstatin. Leupeptin treatment also disrupted SDS-stable dimer formation in T cell clones. These findings indicate that cysteine protease activity is required for successful Class II MHC processing in both B cells and CD4+ T cells.
Figure 2. Although Ii processing in both CD4+ T cells and B cells is leupeptin- dependent, CD4+ T cell clones exhibit a faster rate of αβ:peptide formation and increased Iip24 fragment formation.
A donor-matched EBV-transformed B cell line (A) and CD4+ T cell clone (B) were treated with leupeptin (1mM), pepstatin A (10μM), or control amounts of DMSO before pulse with [35S]-methionine and chase with unlabeled media for the times indicated. HLA-DR complexes and associated Ii fragments were immunoprecipitated and analyzed by SDS-PAGE under denaturing conditions. The class II MHC α and β chains are indicated, as well as the Ii isoforms p41 and p31, and the Ii degradation intermediates p24, p22 and p10. (C) Quantification of Ii fragment formation (mean±SD).
Although we found cysteine protease activity necessary for Class II MHC maturation in both B and T cells, other proteases can cleave Ii and alter the kinetics of class II processing. We observed in some clones the formation of p24 upon leupeptin treatment and reduced formation of p22 with upon treatment with both leupeptin and pepstatin (Fig. 2B, 2C), phenomena that were not observed in donor matched BCL. Cysteine proteases may dominate class II processing in both B cells and T cells, but our data argues for differences in the proteolytic repertoire between these two cellular subsets that could subtly alter class II processing.
AEP does not contribute to Ii processing in CD4+ T cell clones
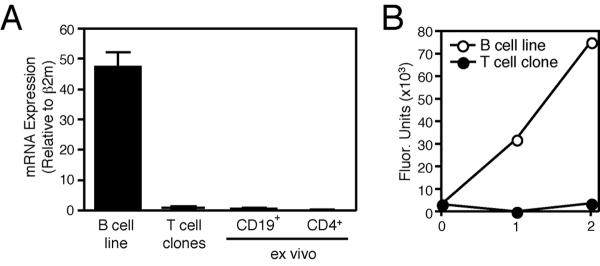
We have previously reported that asparagine endopeptidase inhibition results in development of p24 in leupeptin-treated BCL and loss of p22 in BCL treated with both leupeptin and pepstatin(21). As we observed similar Ii cleavage fragment patterns in CD4+ T cell clones treated with leupeptin and leupeptin/pepstatin (Fig. 3A), we hypothesized that these cells lack AEP activity. Indeed, AEP mRNA was barely detectable in CD4+ T cell clones, as compared to donor matched BCL, or in CD19+ and CD4+ HLA-DR+ T cells ex vivo (Fig. 3A). Furthermore, we could not detect AEP activity in CD4+ T cell clones by direct enzymatic assay (Fig. 3B). These findings show that AEP is not significantly expressed in CD4+ T cells and therefore does not play a role in Class II MHC processing and antigen presentation in these cells.
Figure 3. CD4+ T cells do not express AEP.
(A) Quantification of AEP mRNA in B cell lines, CD4+ T cell clones, and ex vivo CD19+ B cells and CD3+ CD4+ HLA-DR+ T cells isolated from two donors (mean±SD). (B) AEP activity in post-nuclear lysate of donor-matched BCL (B cell) and a CD4+ T cell clone (T cell), as measured by hydrolysis of the synthetic peptide substrate Z-Ala-Ala-Asn-AMC (mean±SD).
Cathepsin S inhibition blocks Ii cleavage in CD4+ T cell clones
CatS plays a critical role in class II MHC processing in murine and human B cells, dendritic cells, and macrophages (17, 26, 27). Given the blockade in invariant chain proteolysis imposed by cysteine protease inhibition in CD4+ T cell clones (Fig. 2B), we hypothesized that CatS activity is required for processing of class II MHC in these non-traditional APC.
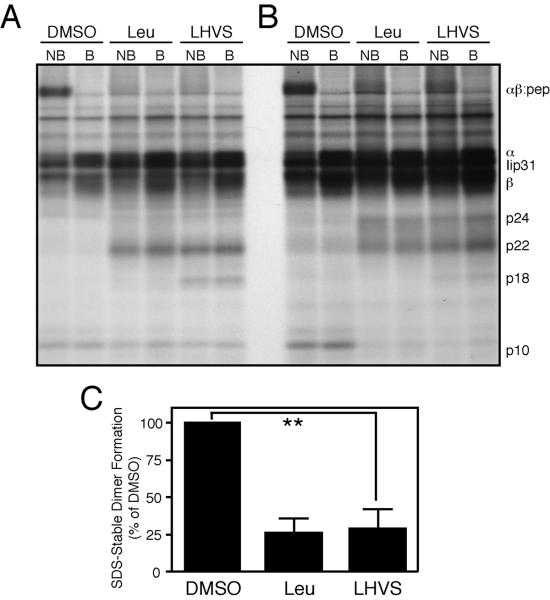
To test this hypothesis, we measured Ii processing and SDS-stable dimer formation in CD4+ T cell clones treated either control amounts of DMSO, leupeptin, or 5nM N-morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl (LHVS) (Fig. 4). We used this low concentration of LHVS to selectively inhibit CatS (17, 28, 29). We pulsed inhibitor-treated T cell clones with [35S]-methionine and then chased the radiolabeled proteins for 6 hours with unlabeled media. After chase, we immunoprecipitated properly folded HLA-DR αβ and Ii complexes from these lysates and resolved both Ii cleavage fragments and SDS-stable dimer formation with SDS-PAGE. As observed previously, treatment with leupeptin resulted in blockade of invariant chain degradation and the formation of the fragments Iip22 and Iip24 (Fig. 4A–B). Leupeptin furthermore significantly reduced the total percentage of SDS-stable dimer formation (to 26.3±8.02%; mean±SD; n=4) (Fig. 4C), confirming a requisite role for cysteine proteases in class II MHC maturation in T cell clones.
Figure 4. Inhibition of cathepsin S blocks nascent αβ:peptide dimer formation in CD4+ T cell clones.
(A–B) CD4+ T cell clones were pulsed with [35S]-methionine and chased for 6 hours after treatment with leupeptin, 5nM LHVS, or control amounts of DMSO. HLA-DR α, β, and associated Ii fragments were then immunoprecipitated, denatured by boiling (where indicated, B), and resolved via SDS-PAGE (NB = non-boiled). A and B are representative autoradiographs of single T cell clones derived from two different healthy donors. C. Quantification of relative SDS-stable dimer formation. (**p= 0.0014; mean,–SEM; n=4 clones from 2 donors).
Similar to treatment with leupeptin, selective inhibition of CatS successfully impaired HLA-DR maturation in both CD4+ T cells and BCL (Fig. 4, data not shown). This treatment resulted in both invariant chain cleavage fragment generation and the reduction of SDS-stable dimers (to 27.5±12.4% SDS-stable dimer formation; mean±SD; n=4)(Fig. 4C). Given the impact of 5nM LHVS treatment on nascent αβ:peptide formation, we conclude that CatS is required for successful Ii cleavage in CD4+ T cells.
Cathepsin S is downregulated in activated CD4+ T cell clones
Dendritic cells, B cells, and γδ+ T cells modulate class II MHC processing and presentation early post-activation (30, 31). To determine the effect of short-term activation on Class II MHC expression in CD4+ T cells, we stimulated T cell clones with PMA and ionomycin or αCD3 and αCD28 for 18 hours and then stained for cell surface HLA-DR (Fig. 5A). At this early timepoint, activated CD4+ T cell clones expressed less HLA-DR than resting clones (down to 34.7±1.0% from 54.7±1.6%; p<0.0001; mean±SEM,; n=35 clones). These findings are consistent with studies showing that PMA treatment of previously activated—and therefore class II MHC+—T cells reduces class II MHC expression on these cells(32).
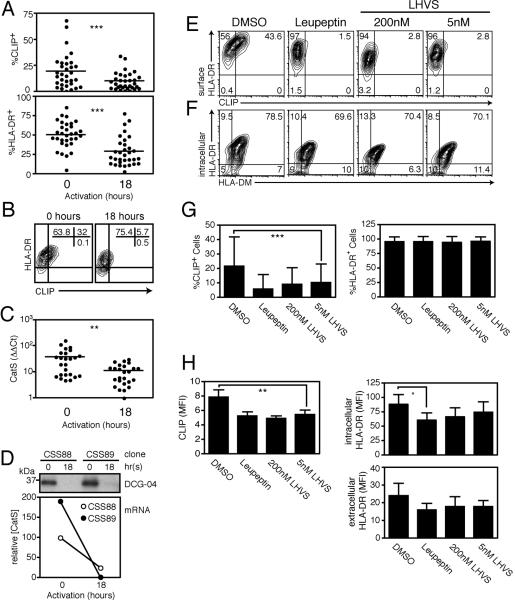
Figure 5. Downregulation of cathepsin S expression and activity decreases cell-surface CLIP expression in recently activated CD4+ T cell clones.
(A–B) Cell surface CLIP and HLA-DR expression in a panel of CD4+ T cell clones from a single donor (A) and in a single representative clone (B), at rest and after 18 hours of mitogenic activation with PMA and ionomycin (***p<0.0001). (C) CatS mRNA expression in a panel of CD4+ T cell clones from a single donor, at rest and after 18 hours of activation (**p=0.0026; relative to BCL expression). (D) Downregulation of Cat S in two representative clones after 18 hours of activation, as indicated by DCG-04 labeling and mRNA expression (relative to β2microglobulin). (E–F) CD4+ T cell clones treated for 18 hours with the pan-cysteine protease inhibitor leupeptin (1mM), 200nM LHVS (CatB/L/S inhibition), 5nM LHVS (CatS alone), or control amounts of DMSO before staining and flow cytometry. (G) Percentage of CLIP positive cells and HLA-DR positive cells after 18 hours of inhibition (***p=0.0007; mean±SD). (H) MFI of CLIP and HLA-DR expression after 18 hours of inhibition (**p=0.0063; *p=0.0365; mean±SD).
The loss of HLA-DR from the cell surface coincides with the reduction of both CatS expression and activity, which were significantly decreased in T cell clones after 18 hours of activation (Fig. 5C–D). Downregulation of cysteine protease expression and activity was restricted to CatS, as CatB and to a lesser extent CatL were upregulated post activation in both clones and HLA-DR+ CD4+ T cells ex vivo (data not shown). As CatS is required for the optimal formation of nascent HLA-DR complexes (Fig. 4), downregulation of this protease could account for the reduction of cell surface Class II MHC post-activation. Such a direct relationship would imply that CatS actively maintains Class II MHC on the cell surface or indicate that T cells rapidly internalize and degrade class II molecules.
To determine the consequence of CatS ablation on HLA-DR expression, we treated CD4+ T cell clones for 18 hours with leupeptin or LHVS and stained for cell surface HLA-DR. Treatment of CD4+ T cell clones with either leupeptin or LHVS was insufficient to reduce the percentage of HLA-DR+ cells in the clones tested (Fig. 5E–G). The mean density of HLA-DR molecules was reduced in some clones after treatment. Significant downregulation of intracellular HLA-DR was observed only in clones treated with leupeptin (from 88.06±15.92 to 60.48±11.3; mean±SEM; n=12; p=0.0365), but not LHVS (65.78±15.6 and 74.48±18.59), and downregulation of extracellular HLA-DR expression was not statistically significant (from 20.42±5.97 to 13.8±3.22; mean±SEM; n=12; p=0.0576) (Fig. 5H). Therefore, short-term cysteine protease inhibition did not directly reduce cell surface HLA-DR expression. Of course continued inhibition of CatS contributes to loss of class II MHC over time because nascent complex formation is blocked (data not shown), but our data indicates that this mechanism cannot by itself account for the observed loss of HLA-DR early post-activation. These results are consistent with the extended half-life of Class II MHC molecules(33) and do not provide evidence for rapid turnover of class II in T cells.
Although short-term cysteine protease blockade did not significantly reduce cell surface HLA-DR expression, LHVS treatment did result in the loss of the invariant chain fragment CLIP from the cell surface (Figure 5E, G, H). Indeed, inhibition of CatS alone with 5nM LHVS reduced expression of CLIP in the HLA-DR binding pocket (from 21.6±0.6% to 10.2±0.4%; p=0.0007; mean±SEM; n=32 clones) (Fig. 5G). Continued treatment with LHVS for 48 hours also resulted in significant downregulation of CLIP (34.7±1.9% control to 7.2±0.5% 5nM LHVS; p=0.0005; mean±SEM; n=12 clones, data not shown), without concomitant loss of total cell surface HLA-DR. As cell surface CLIP was also downregulated early post-activation, even in individual clones with limited total HLA-DR downregulation (Fig. 5B), CatS likely plays a role in the maintenance of CLIP on the cell surface.
CLIP fragments bound in the peptide-binding groove of class II MHC heterodimers are exchanged for antigenic peptide through the action of the loading molecule HLA-DM(12), although CLIP exchange can also occur in the absence of HLA-DM (34, 35). To verify that loss of CLIP from the cell surface was not due to differences in HLA-DM expression, we stained protease inhibitor-treated CD4+ T cell clones for intracellular HLA-DM. HLA-DM expression remained constant, while CLIP expression decreased in these cells (Fig. 5F). Although the possibility remains that changes in HLA-DM localization or kinetic activity could impact peptide editing, there is no evidence to date that protease inhibitors affect such action. We therefore conclude that short-term inhibition of CatS activity in activated CD4+ T cell clones results in downregulation of cell surface CLIP but not reduction of total Class II MHC.
Cathepsins B, L, and S are differentially expressed in CD4+ T cell clones and BCL
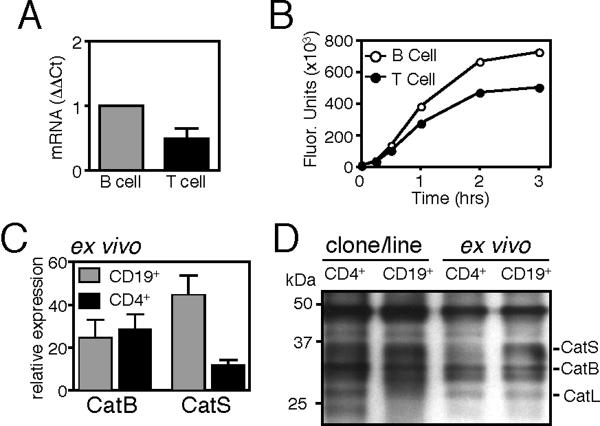
Cysteine proteases other than CatS have been implicated in both invariant chain proteolysis and peptide epitope generation(18, 36, 37). We wished to identify differences in lysosomal protease expression and activity that could contribute to class II MHC processing in CD4+ T cells, as compared to B cells. We focused our work on cathepsins B, L, and S, as these lysosomal cysteine proteases have been implicated in class II MHC processing and presentation (38) and are also expressed in CD4+ T cells (Fig 6A, Fig. 7A). We found that resting CD4+ HLA-DR+ T cell clones express less CatS mRNA (Fig. 6A) and contain less CatS activity (Fig. 6B) than donor-matched BCL. We verified these patterns of expression in cells ex vivo and confirmed that peripheral blood HLA-DR+ CD4+ T cells contained fewer CatS transcripts and less active CatS than CD19+ B cells (Fig. 6C–D).
Figure 6. Cathepsin S expression and activity are reduced in CD4+ T cells, as compared to CD19+ B cells.
(A) CatS mRNA expression in CD4+ T cell clones (T cell) as compared to a donor-matched EBV-transformed B cell line (B cell) (mean±SD). (B) Measurement of CatS enzyme activity in post-nuclear lysates of a CD4+ T cell clone (closed circle) and donor matched B cell line (open circle) as indicated by cleavage of the substrate Bos-Val-Leu-Lys-AMC. (C) CatS and CatB mRNA expression in peripheral blood CD19+ B cells and CD3+CD4+HLA-DR+ T cells (mean±SEM; n=5; relative to β2microglobulin). (D) Cysteine protease activity in a BCL, CD4+ T cell clone, ex vivo CD19+ B cells and CD3+CD4+ T cells from a representative donor, assayed with the activity-based probe DCG-04.
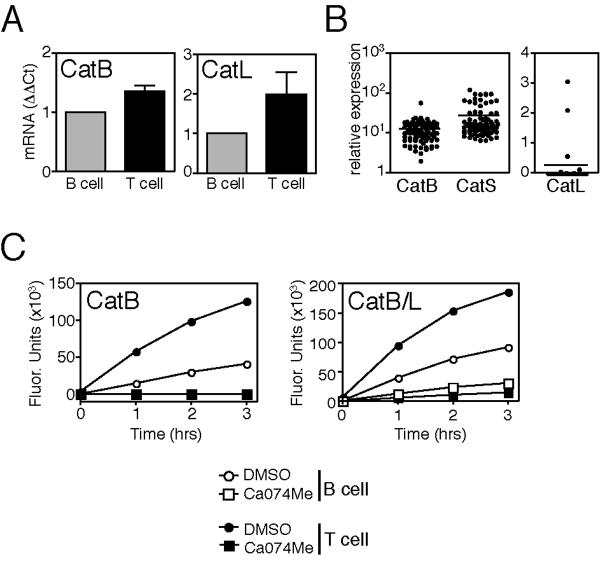
Figure 7. CD4+ T cells express more active cathepsin B and cathepsin L than B cells.
mRNA expression (A) relative to donor-matched BCL and (B) in a panel of clones derived from a single donor. (C) Hydrolysis of the synthetic peptide substrates Z-Arg- Arg-AMC (CatB specific) and Z-Phe-Arg-AMC (CatB/L) in lysates treated with the CatB inhibitor CA074Me (100nM) or with control amounts of DMSO.
Conversely, CD4+ T cell clones expressed more CatB and CatL message and activity than BCL, but this significant difference in expression could not be extended to ex vivo cell populations (Fig. 6C–D, Fig. 7, data not shown). Furthermore, CatL transcripts were only found in a subset of CD4+ T cell clones; lack of CatL mRNA did not correlate with the absence of cell surface HLA-DR on a given clone.
HLA-DR+CD4+ T cells ex vivo express less CLIP than CD19+ T cells
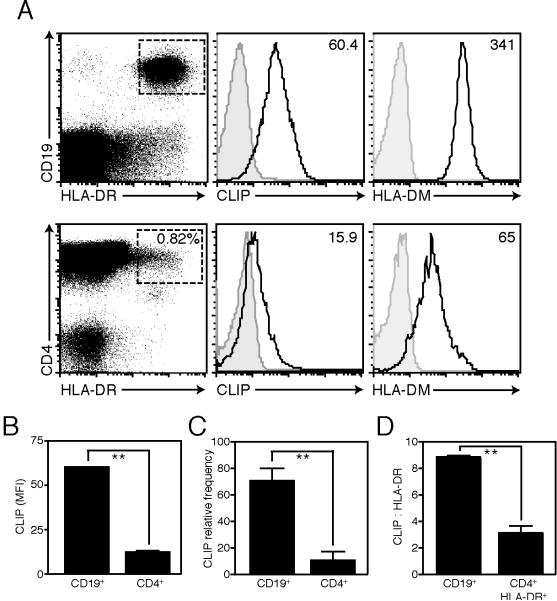
We observed that HLA-DR+CD4+ T cells express less active CatS than CD19+ B cells (Fig 4C–D). In order to determine the consequence of this reduced CatS expression on CLIP presentation in these cells, we stained for cell surface CLIP in peripheral blood. HLA-DR+ CD4+ T cells ex vivo express less cell surface CLIP than HLA-DR+ CD19+ B cells (12.4±0.3 versus 60.4±0.1 CLIP MFI, mean±SEM, n=3) (Fig. 8). The low level of CLIP expression on HLA-DR+ CD4+ T cells was not due to overexpression of HLA-DM in this subset (Fig. 8A). Indeed, intracellular HLA-DM expression was lower in CD4+ T cells than in CD19+ B cells. These data suggests that the modest CatS activity in HLA-DR+ CD4+ T cells is not sufficient to maintain CLIP on the cell surface, while CD19+ B cells, which express higher levels of CatS, constitutively express cell surface CLIP.
Figure 8. Peripheral blood CD4+HLA-DR+ T cells express less CLIP than CD19+ B cells.
(A) Cell surface expression of CLIP and intracellular expression of HLA-DM in peripheral blood mononuclear cells purified from a representative donor, gated on CD3+CD4+HLA-DR+ T cells or CD19+HLA-DR+ B cells (CD3 gate shown for CD4+ cells; MFI indicated; isotype control shown in grey). (B) CLIP mean fluorescence intensity (MFI; **p=0.001; mean±SEM; n=3), (C) relative frequency of CLIP+ cells (expressed as %CLIP+/%HLA-DR+; **p=0.002; mean±SEM; n=5) in CD3+CD4+ T cells or CD19+ B cells ex vivo and (D) the ratio of CLIP to HLA-DR in CD3+CD4+HLA-DR+ T cells versus CD19+B cells (expressed as [CLIP (MFI)/HLA-DR (MFI)]*100; **p=0.0047; mean±SD).
Discussion
HLA-DR expression on CD4+ T cells was observed several decades ago, but the mechanisms of class II MHC proteolytic regulation in these cells remain undefined. With the use of protease inhibitors, we examined the requirements for successful Ii processing and class II MHC complex presentation in constitutively HLA-DR+CD4+ T cell clones. Our results demonstrate that CatS is crucial for Ii proteolysis in these cells. Specific inhibition of CatS with low concentrations of LHVS resulted in the formation of Ii cleavage intermediates and blocked the successful generation of αβ:peptide complexes (Fig. 4).
Furthermore, we find that downregulation of CatS in early-activated HLA-DR+ CD4+ T cells results in the loss of CLIP from the cell surface but does not significantly reduce total class II MHC presentation (Fig. 5). Therefore the prevailing consequence of variable CatS expression is alteration of the peptide repertoire: HLA-DR+ CD4+ T cells both in vitro and ex vivo express less CatS and therefore maintain less CLIP on the cell surface than donor-matched B cells (Fig. 6, 8). A lower level of CatS expression in CD4+ T cells does not necessarily precipitate equivalently low class II MHC expression. It is likely that alternative mechanisms of class II MHC regulation such as complex trafficking and targeted degradation also contribute to expression levels and are differentially active in B cells and T cells. In fact, our data suggests that reduction of class II MHC expression in T cells post-activation is predominantly controlled by a mechanism other than CatS downregulation (Fig. 5).
CatS activity alone does not account for limited expression of Class II MHC within the CD4+ T cell population, as the majority of CD4+ T cells ex vivo do not express the class II transactivator (data not shown). Our data indicates, however, that in CD4+ T cells that do express class II MHC CatS regulates nascent class II MHC complex formation.
HLA-DR+ CD4+ T cells in peripheral blood, while rare, do include a functionally distinct subset of CD25hi FoxP3+ natural regulatory T cells(10) and are upregulated in patients with autoimmune disease(6) and HTLV-1(39). HLA-DR is also expressed on activated T cells, although activation alone is not sufficient to induce HLA-DR expression(10). While the function of Class II MHC on these cells remains uncertain, presentation of class II-restricted antigen by T cells has been implicated in anergy induction (40). Additionally, conserved regions of HLA-DR have been reported to bind the immunoregulatory receptors LAG-3 (CD223) (41) and Tirc7 (42).
Whatever functional role HLA-DR ultimately plays in immune modulation, the ability of this ligand to bind the TCR of CD4+ T cells is axiomatic. Identification of the peptides presented by these cells may lend some insight into the target and functional outcome of T-T presentation. Because HLA-DR+ CD4+ T cells lack any known mechanism for professional antigen acquisition, these cells are believed to present self-peptide or T cell tropic viruses(24, 43). An abundance of endogenous peptide in the MHC binding pocket is not unique to the HLA-DR+ CD4+ T cell. In professional APC, endogenous peptide encompasses a significant fraction of the immune synapse (44). The Ii fragment CLIP is the most predominant self-peptide presented via class II MHC and in human dendritic cells, CLIP-MHC complexes are upregulated following inflammatory stimuli(45). We were therefore surprised to observe that HLA-DR+ CD4+ T cells express less cell surface CLIP than B cells and that CLIP expression decreased following activation (Fig 5, 7). Our findings demonstrate that class II MHC presentation in CD4+ T cells is not restricted to CLIP, and suggest that CLIP expression is differentially regulated in T cells as compared to professional APC. Thus, the repertoire of endogenous protein bound to T cell MHC products may be more complex than has previously been suggested(3).
We identified differences in lysosomal protease expression between T cells and B cells that may lead to the generation of different peptide pools available for class II MHC binding. CD4+ T cells do not express AEP (Fig. 3), the only protease to date shown to be requisite for the generation of an antigenic peptide (46), and can express both CatL and CatV (Fig. 7, data not shown). This proteolytic profile, however, is not dissimilar from that of B cells ex vivo (47). Furthermore, the contribution of these proteases to the self-peptide repertoire remains to be seen.
HLA-DR+ CD4+ T cells are non-traditional APC that may play a role in dampening, rather than promoting, immune activation in the human system via class II MHC. Our work demonstrates that, like professional APC, CD4+ T cells possess the processing machinery required for class II MHC:peptide complex formation and that this machinery is regulated post-activation to modulate peptide presentation. Futhermore, our observations establish CatS as the major Ii processing enzyme in T cells.
Acknowledgements
The authors would like to thank G. Bériou, C. Baecher-Allan, D. Kozoriz, H. Hang, S. Kent, and C. Ashley for advice and technical support.
This work was supported by National Institutes of Health grants.
References
- 1.Evans RL, Faldetta TJ, Humphreys RE, Pratt DM, Yunis EJ, Schlossman SF. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978;148:1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979;150:246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holling TM, Schooten E, van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum Immunol. 2004;65:282–290. doi: 10.1016/j.humimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Hong SC, Hughes CC, Janeway CA, Jr., Flavell RA. CIITA activates the expression of MHC class II genes in mouse T cells. Int Immunol. 1995;7:1515–1518. doi: 10.1093/intimm/7.9.1515. [DOI] [PubMed] [Google Scholar]
- 5.Holling TM, van der Stoep N, Quinten E, van den Elsen PJ. Activated human T cells accomplish MHC class II expression through T cell-specific occupation of class II transactivator promoter III. J Immunol. 2002;168:763–770. doi: 10.4049/jimmunol.168.2.763. [DOI] [PubMed] [Google Scholar]
- 6.Yu DT, Winchester RJ, Fu SM, Gibofsky A, Ko HS, Kunkel HG. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980;151:91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaSalle JM, Ota K, Hafler DA. Presentation of autoantigen by human T cells. J Immunol. 1991;147:774–780. [PubMed] [Google Scholar]
- 8.Wucherpfennig KW, Hollsberg P, Richardson JH, Benjamin D, Hafler DA. T-cell activation by autologous human T-cell leukemia virus type I-infected T-cell clones. Proc Natl Acad Sci U S A. 1992;89:2110–2114. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 1988;334:530–532. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- 10.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 11.Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Curr Opin Immunol. 2004;16:96–102. doi: 10.1016/j.coi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa TY, Rudensky AY. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunological Reviews. 1999;172:121–129. doi: 10.1111/j.1600-065x.1999.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 14.Villadangos JA, Bryant RA, Deussing J, Driessen C, Lennon- Dumenil AM, Riese RJ, Roth W, Saftig P, Shi GP, Chapman HA, Peters C, Ploegh HL. Proteases involved in MHC class II antigen presentation. Immunol Rev. 1999;172:109–120. doi: 10.1111/j.1600-065x.1999.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 15.Brachet V, Raposo G, Amigorena S, Mellman I. Ii chain controls the transport of major histocompatibility complex class II molecules to and from lysosomes. J Cell Biol. 1997;137:51–65. doi: 10.1083/jcb.137.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neefjes JJ, Ploegh HL. Inhibition of endosomal proteolytic activity by leupeptin blocks surface expression of MHC class II molecules and their conversion to SDS resistance alpha beta heterodimers in endosomes. EMBO J. 1992;11:411–416. doi: 10.1002/j.1460-2075.1992.tb05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riese RJ, Wolf PR, Brömme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Rudensky A. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998:4. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 19.Shi GP, Bryant RA, Riese R, Verhelst S, Driessen C, Li Z, Bromme D, Ploegh HL, Chapman HA. Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J Exp Med. 2000;191:1177–1186. doi: 10.1084/jem.191.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biddison WE. Generation of continuously growing B cell lines by epstein-barr virus transformation. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb0204s01. Chapter 2:Unit 2 4. [DOI] [PubMed] [Google Scholar]
- 21.Costantino CM, Hang HC, Kent SC, Hafler DA, Ploegh HL. Lysosomal cysteine and aspartic proteases are heterogeneously expressed and act redundantly to initiate human invariant chain degradation. J Immunol. 2008;180:2876–2885. doi: 10.4049/jimmunol.180.5.2876. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura H, Kamon H, Sawa S, Park S, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 Controls Intracellular MHC Class II αβ Dimer Level through Cathepsin S Activity in Dendritic Cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Maehr R, Hang HC, Mintern JD, Kim YM, Cuvillier A, Nishimura M, Yamada K, Shirahama-Noda K, Hara-Nishimura I, Ploegh HL. Asparagine endopeptidase is not essential for class II MHC antigen presentation but is required for processing of cathepsin L in mice. J Immunol. 2005;174:7066–7074. doi: 10.4049/jimmunol.174.11.7066. [DOI] [PubMed] [Google Scholar]
- 24.Hewitt CR, Feldmann M. Human T cell clones present antigen. J Immunol. 1989;143:762–769. [PubMed] [Google Scholar]
- 25.Matsui Y, Shapiro HM, Sheehy MJ, Christenson L, Staunton DE, Eynon EE, Yunis EJ. Differential expression of T cell differentiation antigens and major histocompatibility antigens on activated T cells during the cell cycle. Eur J Immunol. 1986;16:248–251. doi: 10.1002/eji.1830160307. [DOI] [PubMed] [Google Scholar]
- 26.Beck H, Schwarz G, Schroter CJ, Deeg M, Baier D, Stevanovic S, Weber E, Driessen C, Kalbacher H. Cathepsin S and an asparagine-specific endoprotease dominate the proteolytic processing of human myelin basic protein in vitro. Eur J Immunol. 2001;31:3726–3736. doi: 10.1002/1521-4141(200112)31:12<3726::aid-immu3726>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Riese RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, De Sanctis GT, Ploegh HL, Chapman HA. Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest. 1998;101:2351–2363. doi: 10.1172/JCI1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer JT, Rasnick D, Klaus JL, Bromme D. Vinyl sulfones as mechanism-based cysteine protease inhibitors. J Med Chem. 1995;38:3193–3196. doi: 10.1021/jm00017a002. [DOI] [PubMed] [Google Scholar]
- 30.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 31.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 32.Oshima S, Eckels DD. Selective signal transduction through the CD3 or CD2 complex is required for class II MHC expression by human T cells. J Immunol. 1990;145:4018–4025. [PubMed] [Google Scholar]
- 33.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 34.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 35.Martin WD, Hicks GG, Mendiratta SK, Leva HI, Ruley HE, Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 36.Avva RR, Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994;1:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 37.Mizuochi T, Yee ST, Kasai M, Kakiuchi T, Muno D, Kominami E. Both cathepsin B and cathepsin D are necessary for processing of ovalbumin as well as for degradation of class II MHC invariant chain. Immunol Lett. 1994;43:189–193. doi: 10.1016/0165-2478(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 38.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 39.Akari H, Goto Y, Shinjo T. Detection of the cellular membrane proteins on human T cell leukemia virus type I. Arch Virol. 1995;140:375–382. doi: 10.1007/BF01309871. [DOI] [PubMed] [Google Scholar]
- 40.LaSalle JM, Tolentino PJ, Freeman GJ, Nadler LM, Hafler DA. Early signaling defects in human T cells anergized by T cell presentation of autoantigen. J Exp Med. 1992;176:177–186. doi: 10.1084/jem.176.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macon-Lemaitre L, Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology. 2005;115:170–178. doi: 10.1111/j.1365-2567.2005.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulwin GC, Walter S, Schlawinsky M, Heinemann T, Schulze A, Hohne W, Krause G, Kalka-Moll W, Fraser P, Volk HD, Lohler J, Milford EL, Utku N. HLA-DR alpha 2 mediates negative signalling via binding to Tirc7 leading to anti-inflammatory and apoptotic effects in lymphocytes in vitro and in vivo. PLoS ONE. 2008;3:e1576. doi: 10.1371/journal.pone.0001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanzavecchia A, Abrignani S, Scheidegger D, Obrist R, Dorken B, Moldenhauer G. Antibodies as antigens. The use of mouse monoclonal antibodies to focus human T cells against selected targets. J Exp Med. 1988;167:345–352. doi: 10.1084/jem.167.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krogsgaard M, Juang J, Davis MM. A role for “self” in T-cell activation. Semin Immunol. 2007;19:236–244. doi: 10.1016/j.smim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Röhn T, Boes M, Wolters D, Spindeldreher S, Müller B, Langen H, Ploegh H, Vogt A, Kropshofer H. Upregulation of the CLIP self peptide on mature dendritic cells antagonizes T helper type 1 polarization. Nat Immunol. 2004;5:909–918. doi: 10.1038/ni1108. [DOI] [PubMed] [Google Scholar]
- 46.Manoury B, Hewitt EW, Morrice N, Dando PM, Barrett AJ, Watts C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 1998;396:695–699. doi: 10.1038/25379. [DOI] [PubMed] [Google Scholar]
- 47.Burster T, Beck A, Tolosa E, Marin-Esteban V, Rotzschke O, Falk K, Lautwein A, Reich M, Brandenburg J, Schwarz G, Wiendl H, Melms A, Lehmann R, Stevanovic S, Kalbacher H, Driessen C. Cathepsin G, and not the asparagine-specific endoprotease, controls the processing of myelin basic protein in lysosomes from human B lymphocytes. J Immunol. 2004;172:5495–5503. doi: 10.4049/jimmunol.172.9.5495. [DOI] [PubMed] [Google Scholar]